From mouse to man: safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH-F3+GLA-SE
- PMID: 26175894
- PMCID: PMC4488838
- DOI: 10.1038/cti.2015.6
From mouse to man: safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH-F3+GLA-SE
Abstract
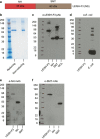
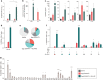
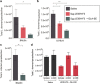
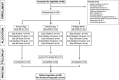
Key antigens of Leishmania species identified in the context of host responses in Leishmania-exposed individuals from disease-endemic areas were prioritized for the development of a subunit vaccine against visceral leishmaniasis (VL), the most deadly form of leishmaniasis. Two Leishmania proteins-nucleoside hydrolase and a sterol 24-c-methyltransferase, each of which are protective in animal models of VL when properly adjuvanted- were produced as a single recombinant fusion protein NS (LEISH-F3) for ease of antigen production and broad coverage of a heterogeneous major histocompatibility complex population. When formulated with glucopyranosyl lipid A-stable oil-in-water nanoemulsion (GLA-SE), a Toll-like receptor 4 TH1 (T helper 1) promoting nanoemulsion adjuvant, the LEISH-F3 polyprotein induced potent protection against both L. donovani and L. infantum in mice, measured as significant reductions in liver parasite burdens. A robust immune response to each component of the vaccine with polyfunctional CD4 TH1 cell responses characterized by production of antigen-specific interferon-γ, tumor necrosis factor and interleukin-2 (IL-2), and low levels of IL-5 and IL-10 was induced in immunized mice. We also demonstrate that CD4 T cells, but not CD8 T cells, are sufficient for protection against L. donovani infection in immunized mice. Based on the sum of preclinical data, we prepared GMP materials and performed a phase 1 clinical study with LEISH-F3+GLA-SE in healthy, uninfected adults in the United States. The vaccine candidate was shown to be safe and induced a strong antigen-specific immune response, as evidenced by cytokine and immunoglobulin subclass data. These data provide a strong rationale for additional trials in Leishmania-endemic countries in populations vulnerable to VL.
Figures

Similar articles
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Leishmania donovani Nucleoside Hydrolase (NH36) Domains Induce T-Cell Cytokine Responses in Human Visceral Leishmaniasis.Front Immunol. 2017 Mar 7;8:227. doi: 10.3389/fimmu.2017.00227. eCollection 2017. Front Immunol. 2017. PMID: 28321221 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
The effectiveness of abstinence-based and harm reduction-based interventions in reducing problematic substance use in adults who are experiencing homelessness in high income countries: A systematic review and meta-analysis: A systematic review.Campbell Syst Rev. 2024 Apr 21;20(2):e1396. doi: 10.1002/cl2.1396. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38645303 Free PMC article. Review.
Cited by
-
Current leishmaniasis drug discovery.RSC Med Chem. 2022 Aug 26;13(9):1029-1043. doi: 10.1039/d1md00362c. eCollection 2022 Sep 21. RSC Med Chem. 2022. PMID: 36324493 Free PMC article. Review.
-
A Review of Leishmaniasis: Current Knowledge and Future Directions.Curr Trop Med Rep. 2021;8(2):121-132. doi: 10.1007/s40475-021-00232-7. Epub 2021 Mar 17. Curr Trop Med Rep. 2021. PMID: 33747716 Free PMC article. Review.
-
Post-Genomics and Vaccine Improvement for Leishmania.Front Microbiol. 2016 Apr 6;7:467. doi: 10.3389/fmicb.2016.00467. eCollection 2016. Front Microbiol. 2016. PMID: 27092123 Free PMC article. Review.
-
The adjuvant GLA-AF enhances human intradermal vaccine responses.Sci Adv. 2018 Sep 12;4(9):eaas9930. doi: 10.1126/sciadv.aas9930. eCollection 2018 Sep. Sci Adv. 2018. PMID: 30221194 Free PMC article. Clinical Trial.
-
Immunotherapy and immunochemotherapy in combating visceral leishmaniasis.Front Med (Lausanne). 2023 May 17;10:1096458. doi: 10.3389/fmed.2023.1096458. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37265481 Free PMC article. Review.
References
-
- Kedzierski L, Sakthianandeswaren A, Curtis JM, Andrews PC, Junk PC, Kedzierska K. Leishmaniasis: current treatment and prospects for new drugs and vaccines. Curr Med Chem. 2009;16:599–614. - PubMed
-
- World Health Organization Control of the leishmaniases. World Health Organ Tech Rep Ser. 2010;793:1–158. - PubMed
-
- Joshi AB, Banjara MR, Pokhrel S, Jimba M, Singhasivanon P, Ashford RW. Elimination of visceral leishmaniasis in Nepal: pipe-dreams and possibilities. Kathmandu Univ Med J (KUMJ) 2006;4:488–496. - PubMed
-
- Kumar V, Kesari S, Dinesh DS, Tiwari AK, Kumar AJ, Kumar R, et al. A report on the indoor residual spraying (IRS) in the control of Phlebotomus argentipes, the vector of visceral leishmaniasis in Bihar (India): an initiative towards total elimination targeting 2015 (Series-1) J Vector Borne Dis. 2009;46:225–229. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
