Attenuation of Colitis by Lactobacillus casei BL23 Is Dependent on the Dairy Delivery Matrix
- PMID: 26162873
- PMCID: PMC4542224
- DOI: 10.1128/AEM.01360-15
Attenuation of Colitis by Lactobacillus casei BL23 Is Dependent on the Dairy Delivery Matrix
Abstract
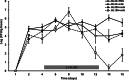
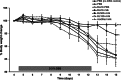
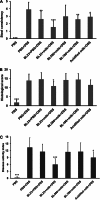
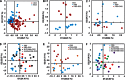
The role of the food delivery matrix in probiotic performance in the intestine is not well understood. Because probiotics are often provided to consumers in dairy products, we investigated the contributions of milk to the health-benefiting performance of Lactobacillus casei BL23 in a dextran sulfate sodium (DSS)-induced murine model of ulcerative colitis. L. casei BL23 protected against the development of colitis when ingested in milk but not in a nutrient-free buffer simulating consumption as a nutritional supplement. Consumption of (acidified) milk alone also provided some protection against weight loss and intestinal inflammation but was not as effective as L. casei and milk in combination. In contrast, L. casei mutants deficient in DltD (lipoteichoic acid d-alanine transfer protein) or RecA (recombinase A) were unable to protect against DSS-induced colitis, even when consumed in the presence of milk. Mice fed either L. casei or milk contained reduced quantities of colonic proinflammatory cytokines, indicating that the L. casei DltD(-) and RecA(-) mutants as well as L. casei BL23 in nutrient-free buffer were effective at modulating immune responses. However, there was not a direct correlation between colitis and quantities of these cytokines at the time of sacrifice. Identification of the cecal microbiota by 16S rRNA gene sequencing showed that L. casei in milk enriched for Comamonadaceae and Bifidobacteriaceae; however, the consumption of neither L. casei nor milk resulted in the restoration of the microbiota to resemble that of healthy animals. These findings strongly indicate that probiotic strain efficacy can be influenced by the food/supplement delivery matrix.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Milk and Lacticaseibacillus paracasei BL23 effects on intestinal responses in a murine model of colitis.Am J Physiol Gastrointest Liver Physiol. 2024 Jun 1;326(6):G659-G675. doi: 10.1152/ajpgi.00259.2023. Epub 2024 Apr 9. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 38591132
-
Lactobacillus casei Low-Temperature, Dairy-Associated Proteome Promotes Persistence in the Mammalian Digestive Tract.J Proteome Res. 2015 Aug 7;14(8):3136-47. doi: 10.1021/acs.jproteome.5b00387. Epub 2015 Jul 24. J Proteome Res. 2015. PMID: 26148687
-
Short communication: effect of milk and milk containing Lactobacillus casei on the intestinal microbiota of mice.J Dairy Sci. 2014;97(4):2049-55. doi: 10.3168/jds.2013-7477. Epub 2014 Feb 6. J Dairy Sci. 2014. PMID: 24508432
-
[Bacteria of Lactobacillus casei group: characterization, viability as probiotic in food products and their importance for human health].Arch Latinoam Nutr. 2007 Dec;57(4):373-80. Arch Latinoam Nutr. 2007. PMID: 18524322 Review. Portuguese.
-
Applications of probiotics.Meded Rijksuniv Gent Fak Landbouwkd Toegep Biol Wet. 2001;66(3b):557-61. Meded Rijksuniv Gent Fak Landbouwkd Toegep Biol Wet. 2001. PMID: 15954653 Review.
Cited by
-
The effects of dairy on the gut microbiome and symptoms in gastrointestinal disease cohorts: a systematic review.Gut Microbiome (Camb). 2024 May 7;5:e5. doi: 10.1017/gmb.2024.2. eCollection 2024. Gut Microbiome (Camb). 2024. PMID: 39290657 Free PMC article. Review.
-
Milk and Lacticaseibacillus paracasei BL23 effects on intestinal responses in a murine model of colitis.Am J Physiol Gastrointest Liver Physiol. 2024 Jun 1;326(6):G659-G675. doi: 10.1152/ajpgi.00259.2023. Epub 2024 Apr 9. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 38591132
-
Draft Genome Sequence and Comparative Genome Analysis Reveal Potential Functional Properties in Lacticaseibacillus paracasei ItalPN16.Curr Microbiol. 2023 Nov 1;80(12):399. doi: 10.1007/s00284-023-03515-6. Curr Microbiol. 2023. PMID: 37910267
-
Cell-Free Supernatant Derived from a Lactobacillus casei BL23 Culture Modifies the Antiviral and Immunomodulatory Capacity of Mesenchymal Stromal Cells.Biomedicines. 2023 May 24;11(6):1521. doi: 10.3390/biomedicines11061521. Biomedicines. 2023. PMID: 37371616 Free PMC article.
-
Next-Generation Probiotics: Microflora Intervention to Human Diseases.Biomed Res Int. 2022 Nov 16;2022:5633403. doi: 10.1155/2022/5633403. eCollection 2022. Biomed Res Int. 2022. PMID: 36440358 Free PMC article. Review.
References
-
- Sanders ME, Klaenhammer TR, Ouwehand AC, Pot B, Johansen E, Heimbach JT, Marco ML, Tennila J, Ross RP, Franz C, Page N, Pridmore RD, Leyer G, Salminen S, Charbonneau D, Call E, Lenoir-Wijnkoop I. 2014. Effects of genetic, processing, or product formulation changes on efficacy and safety of probiotics. Ann N Y Acad Sci 1309:1–18. doi:10.1111/nyas.12363. - DOI - PubMed
-
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. 2014. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. doi:10.1038/nrgastro.2014.66. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

