Crucial roles of RSK in cell motility by catalysing serine phosphorylation of EphA2
- PMID: 26158630
- PMCID: PMC4510653
- DOI: 10.1038/ncomms8679
Crucial roles of RSK in cell motility by catalysing serine phosphorylation of EphA2
Abstract
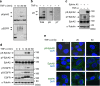
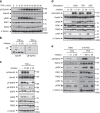
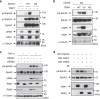
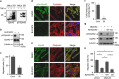
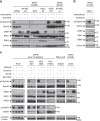
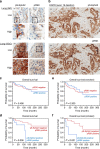
Crosstalk between inflammatory signalling pathways and receptor tyrosine kinases has been revealed as an indicator of cancer malignant progression. In the present study, we focus on EphA2 receptor tyrosine kinase, which is overexpressed in many human cancers. It has been reported that ligand-independent phosphorylation of EphA2 at Ser-897 is induced by Akt. We show that inflammatory cytokines promote RSK-, not Akt-, dependent phosphorylation of EphA2 at Ser-897. In addition, the RSK-EphA2 signalling pathway controls cell migration and invasion of metastatic breast cancer cells. Moreover, Ser-897-phosphorylated EphA2 co-localizes with phosphorylated active form of RSK in various human tumour specimens, and this double positivity is related to poor survival in lung cancer patients, especially those with a smoking history. Taken together, these results indicate that the phosphorylation of EphA2 at Ser-897 is controlled by RSK and the RSK-EphA2 axis might contribute to cell motility and promote tumour malignant progression.
Figures

Similar articles
-
Cellular stress induces non-canonical activation of the receptor tyrosine kinase EphA2 through the p38-MK2-RSK signaling pathway.J Biol Chem. 2023 May;299(5):104699. doi: 10.1016/j.jbc.2023.104699. Epub 2023 Apr 12. J Biol Chem. 2023. PMID: 37059179 Free PMC article.
-
EphA2 is a key effector of the MEK/ERK/RSK pathway regulating glioblastoma cell proliferation.Cell Signal. 2016 Aug;28(8):937-45. doi: 10.1016/j.cellsig.2016.04.009. Epub 2016 Apr 28. Cell Signal. 2016. PMID: 27132626
-
RSK-Mediated Non-canonical Activation of EphA2 by Tamoxifen.Biol Pharm Bull. 2022;45(2):162-168. doi: 10.1248/bpb.b21-00567. Biol Pharm Bull. 2022. PMID: 35110502
-
Emerging and Diverse Functions of the EphA2 Noncanonical Pathway in Cancer Progression.Biol Pharm Bull. 2017;40(10):1616-1624. doi: 10.1248/bpb.b17-00446. Biol Pharm Bull. 2017. PMID: 28966234 Review.
-
[Non-canonical Activation of Receptor Tyrosine Kinases in Cancer Progression].Yakugaku Zasshi. 2017;137(2):141-144. doi: 10.1248/yakushi.16-00229-4. Yakugaku Zasshi. 2017. PMID: 28154322 Review. Japanese.
Cited by
-
Ginsenoside Rg5 inhibits cancer cell migration by inhibiting the nuclear factor-κB and erythropoietin-producing hepatocellular receptor A2 signaling pathways.Oncol Lett. 2021 Jun;21(6):452. doi: 10.3892/ol.2021.12713. Epub 2021 Apr 8. Oncol Lett. 2021. PMID: 33907562 Free PMC article.
-
Oncogenic functions and therapeutic targeting of EphA2 in cancer.Oncogene. 2021 Apr;40(14):2483-2495. doi: 10.1038/s41388-021-01714-8. Epub 2021 Mar 8. Oncogene. 2021. PMID: 33686241 Free PMC article. Review.
-
Matrix Rigidity Controls Epithelial-Mesenchymal Plasticity and Tumor Metastasis via a Mechanoresponsive EPHA2/LYN Complex.Dev Cell. 2020 Aug 10;54(3):302-316.e7. doi: 10.1016/j.devcel.2020.05.031. Epub 2020 Jun 22. Dev Cell. 2020. PMID: 32574556 Free PMC article.
-
Discovery of a sugar-based nanoparticle universally existing in boiling herbal water extracts and their immunostimulant effect.Biochem Biophys Rep. 2018 Oct 11;16:62-68. doi: 10.1016/j.bbrep.2018.08.004. eCollection 2018 Dec. Biochem Biophys Rep. 2018. PMID: 30338298 Free PMC article.
-
Caught in the "Akt": Cross-talk between EphA2 and EGFR through the Akt-PIKfyve axis maintains cellular sensitivity to EGF.Sci Signal. 2018 Jul 31;11(541):eaau1207. doi: 10.1126/scisignal.aau1207. Sci Signal. 2018. PMID: 30065027 Free PMC article. Review.
References
-
- Bennasroune A., Gardin A., Aunis D., Cremel G. & Hubert P. Tyrosine kinase receptors as attractive targets of cancer therapy. Crit. Rev. Oncol. Hematol. 50, 23–38 (2004). - PubMed
-
- Templeton A. J. et al. Prognostic relevance of receptor tyrosine kinase expression in breast cancer: a meta-analysis. Cancer Treat. Rev. 40, 1048–1055 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

