THY-1 Cell Surface Antigen (CD90) Has an Important Role in the Initial Stage of Human Cytomegalovirus Infection
- PMID: 26147640
- PMCID: PMC4492587
- DOI: 10.1371/journal.ppat.1004999
THY-1 Cell Surface Antigen (CD90) Has an Important Role in the Initial Stage of Human Cytomegalovirus Infection
Abstract
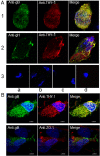
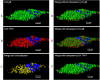
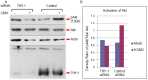
Human cytomegalovirus (HCMV) infects about 50% of the US population, is the leading infectious cause of birth defects, and is considered the most important infectious agent in transplant recipients. The virus infects many cell types in vivo and in vitro. While previous studies have identified several cellular proteins that may function at early steps of infection in a cell type dependent manner, the mechanism of virus entry is still poorly understood. Using a computational biology approach, correlating gene expression with virus infectivity in 54 cell lines, we identified THY-1 as a putative host determinant for HCMV infection in these cells. With a series of loss-of-function, gain-of-function and protein-protein interaction analyses, we found that THY-1 mediates HCMV infection at the entry step and is important for infection that occurs at a low m.o.i. THY-1 antibody that bound to the cell surface blocked HCMV during the initial 60 minutes of infection in a dose-dependent manner. Down-regulation of THY-1 with siRNA impaired infectivity occurred during the initial 60 minutes of inoculation. Both THY-1 antibody and siRNA inhibited HCMV-induced activation of the PI3-K/Akt pathway required for entry. Soluble THY-1 protein blocked HCMV infection during, but not after, virus internalization. Expression of exogenous THY-1 enhanced entry in cells expressing low levels of the protein. THY-1 interacted with HCMV gB and gH and may form a complex important for entry. However, since gB and gH have previously been shown to interact, it is uncertain if THY-1 directly binds to both of these proteins. Prior observations that THY-1 (a) interacts with αVβ3 integrin and recruits paxillin (implicated in HCMV entry), (b) regulates leukocyte extravasation (critical for HCMV viremia), and (c) is expressed on many cells targeted for HCMV infection including epithelial and endothelial cells, fibroblast, and CD34+/CD38- stem cells, all support a role for THY-1 as an HCMV entry mediator in a cell type dependent manner. THY-1 may function through a complex setting, that would include viral gB and gH, and other cellular factors, thus links virus entry with signaling in host cells that ultimately leads to virus infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
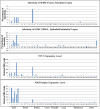
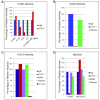
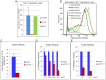
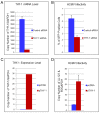

Similar articles
-
Cell Surface THY-1 Contributes to Human Cytomegalovirus Entry via a Macropinocytosis-Like Process.J Virol. 2016 Oct 14;90(21):9766-9781. doi: 10.1128/JVI.01092-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27558416 Free PMC article.
-
Human Cytomegalovirus gH/gL/gO Promotes the Fusion Step of Entry into All Cell Types, whereas gH/gL/UL128-131 Broadens Virus Tropism through a Distinct Mechanism.J Virol. 2015 Sep;89(17):8999-9009. doi: 10.1128/JVI.01325-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085146 Free PMC article.
-
Human Cytomegalovirus Glycoprotein-Initiated Signaling Mediates the Aberrant Activation of Akt.J Virol. 2020 Jul 30;94(16):e00167-20. doi: 10.1128/JVI.00167-20. Print 2020 Jul 30. J Virol. 2020. PMID: 32493823 Free PMC article.
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
-
Human cytomegalovirus tropism for endothelial/epithelial cells: scientific background and clinical implications.Rev Med Virol. 2010 May;20(3):136-55. doi: 10.1002/rmv.645. Rev Med Virol. 2010. PMID: 20084641 Review.
Cited by
-
Virus-host protein interactions as footprints of human cytomegalovirus replication.Curr Opin Virol. 2022 Feb;52:135-147. doi: 10.1016/j.coviro.2021.11.016. Epub 2021 Dec 16. Curr Opin Virol. 2022. PMID: 34923282 Free PMC article. Review.
-
High-Risk Oncogenic Human Cytomegalovirus.Viruses. 2022 Nov 7;14(11):2462. doi: 10.3390/v14112462. Viruses. 2022. PMID: 36366560 Free PMC article. Review.
-
Interferon-inducible LY6E Protein Promotes HIV-1 Infection.J Biol Chem. 2017 Mar 17;292(11):4674-4685. doi: 10.1074/jbc.M116.755819. Epub 2017 Jan 27. J Biol Chem. 2017. PMID: 28130445 Free PMC article.
-
CD147 Promotes Entry of Pentamer-Expressing Human Cytomegalovirus into Epithelial and Endothelial Cells.mBio. 2018 May 8;9(3):e00781-18. doi: 10.1128/mBio.00781-18. mBio. 2018. PMID: 29739904 Free PMC article.
-
Analysis of Cytomegalovirus Glycoprotein and Cellular Receptor Interactions.Methods Mol Biol. 2021;2244:199-211. doi: 10.1007/978-1-0716-1111-1_10. Methods Mol Biol. 2021. PMID: 33555588
References
-
- Mocarski ES, Shenk T, Pass RF (2007) Cytomegaloviruses In: Knipe DMPMH, editor. Fields Virology. Philadelphia, PA: Lippincott, Williams & Wilikins; pp. 2704–2773.
-
- Sinzger C, Grefte A, Plachter B, Gouw AS, The TH, et al. (1995) Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J Gen Virol 76 (Pt 4): 741–750. - PubMed
-
- Compton T, Feire A (2007) Early events in human cytomegalovirus infection. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B et al., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

