Safety and immunologic correlates of Melanoma GVAX, a GM-CSF secreting allogeneic melanoma cell vaccine administered in the adjuvant setting
- PMID: 26143264
- PMCID: PMC4491237
- DOI: 10.1186/s12967-015-0572-3
Safety and immunologic correlates of Melanoma GVAX, a GM-CSF secreting allogeneic melanoma cell vaccine administered in the adjuvant setting
Abstract
Background: Limited adjuvant treatment options exist for patients with high-risk surgically resected melanoma. This first-in-human study investigated the safety, tolerability and immunologic correlates of Melanoma GVAX, a lethally irradiated granulocyte-macrophage colony stimulating factor (GM-CSF)-secreting allogeneic whole-cell melanoma vaccine, administered in the adjuvant setting.
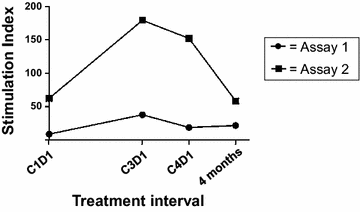
Methods: Patients with stage IIB-IV melanoma were enrolled following complete surgical resection. Melanoma GVAX was administered intradermally once every 28 days for four cycles, at 5E7 cells/cycle (n = 3), 2E8 cells/cycle (n = 9), or 2E8 cells/cycle preceded by cyclophosphamide 200 mg/m(2) to deplete T regulatory cells (Tregs; n = 8). Blood was collected before each vaccination and at 4 and 6 months after treatment initiation for immunologic studies. Vaccine injection site biopsies and additional blood samples were obtained 2 days after the 1st and 4th vaccines.
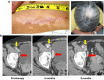
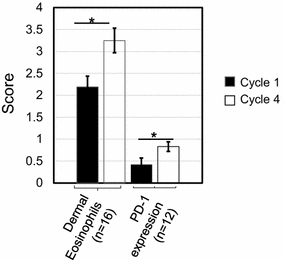
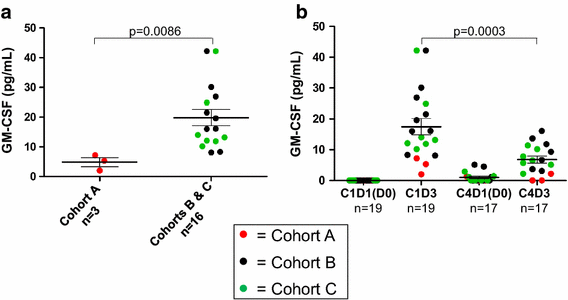
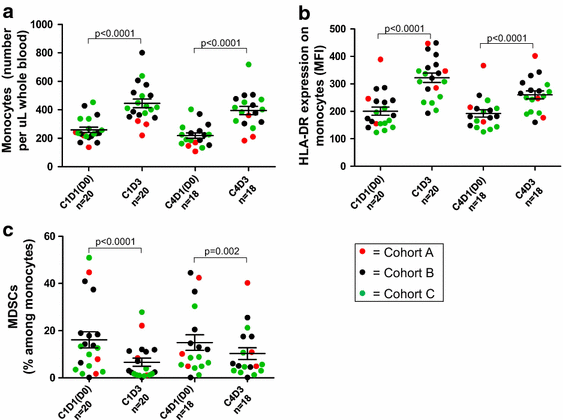
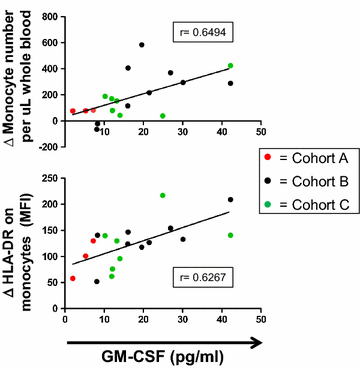
Results: Among 20 treated patients, 18 completed 4 vaccinations. Minimal treatment-related toxicity was observed. One patient developed vitiligo and patches of white hair during the treatment and follow-up period. Vaccine site biopsies demonstrated complex inflammatory infiltrates, including significant increases in eosinophils and PD-1+ lymphocytes from cycle 1 to cycle 4 (P < 0.05). Serum GM-CSF concentrations increased significantly in a dose-dependent manner 48 h after vaccination (P = 0.0086), accompanied by increased numbers of activated circulating monocytes (P < 0.0001) and decreased percentages of myeloid-derived suppressor cells among monocytes (CD14+ , CD11b+ , HLA-DR low or negative; P = 0.002). Cyclophosphamide did not affect numbers of circulating Tregs. No significant changes in anti-melanoma immunity were observed in peripheral T cells by interferon-gamma ELIPSOT, or immunoglobulins by serum Western blotting.
Conclusion: Melanoma GVAX was safe and tolerable in the adjuvant setting. Pharmacodynamic testing revealed complex vaccine site immune infiltrates and an immune-reactive profile in circulating monocytic cell subsets. These findings support the optimization of Melanoma GVAX with additional monocyte and dendritic cell activators, and the potential development of combinatorial treatment regimens with synergistic agents.
Trial registration: NCT01435499.
Figures

Similar articles
-
Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells.J Clin Oncol. 2003 Nov 1;21(21):4016-26. doi: 10.1200/JCO.2003.10.005. J Clin Oncol. 2003. PMID: 14581425 Clinical Trial.
-
Adjuvant therapeutic vaccination in patients with non-small cell lung cancer made lymphopenic and reconstituted with autologous PBMC: first clinical experience and evidence of an immune response.J Transl Med. 2007 Sep 14;5:43. doi: 10.1186/1479-5876-5-43. J Transl Med. 2007. PMID: 17868452 Free PMC article. Clinical Trial.
-
Granulocyte-macrophage-colony-stimulating factor added to a multipeptide vaccine for resected Stage II melanoma.Cancer. 2003 Jan 1;97(1):186-200. doi: 10.1002/cncr.11045. Cancer. 2003. PMID: 12491520 Clinical Trial.
-
Enhancing the clinical activity of granulocyte-macrophage colony-stimulating factor-secreting tumor cell vaccines.Immunol Rev. 2008 Apr;222:287-98. doi: 10.1111/j.1600-065X.2008.00618.x. Immunol Rev. 2008. PMID: 18364009 Review.
-
Vaccines in cancer: GVAX, a GM-CSF gene vaccine.Expert Rev Vaccines. 2005 Jun;4(3):259-74. doi: 10.1586/14760584.4.3.259. Expert Rev Vaccines. 2005. PMID: 16026242 Review.
Cited by
-
The Road Ahead in Pancreatic Cancer: Emerging Trends and Therapeutic Prospects.Biomedicines. 2024 Sep 2;12(9):1979. doi: 10.3390/biomedicines12091979. Biomedicines. 2024. PMID: 39335494 Free PMC article. Review.
-
Current clinical trials for melanoma vaccines: where do we stand?Melanoma Manag. 2016 Dec;3(4):255-257. doi: 10.2217/mmt-2016-0009. Epub 2016 Nov 30. Melanoma Manag. 2016. PMID: 30190896 Free PMC article. No abstract available.
-
Immune-related adverse events with immune checkpoint inhibitors affecting the skeleton: a seminal case series.J Immunother Cancer. 2018 Oct 11;6(1):104. doi: 10.1186/s40425-018-0417-8. J Immunother Cancer. 2018. PMID: 30305172 Free PMC article.
-
Re-Emergence of Dendritic Cell Vaccines for Cancer Treatment.Trends Cancer. 2018 Feb;4(2):119-137. doi: 10.1016/j.trecan.2017.12.007. Trends Cancer. 2018. PMID: 29458962 Free PMC article. Review.
-
In situ vaccination via tissue-targeted cDC1 expansion enhances the immunogenicity of chemoradiation and immunotherapy.J Clin Invest. 2024 Jan 2;134(1):e171621. doi: 10.1172/JCI171621. J Clin Invest. 2024. PMID: 37917174 Free PMC article.
References
-
- Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. - PubMed
-
- Kirkwood JM, Ibrahim JG, Sondak VK, Richards J, Flaherty LE, Ernstoff MS, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444–2458. - PubMed
-
- Kirkwood JM, Ibrahim JG, Sosman JA, Sondak VK, Agarwala SS, Ernstoff MS, et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370–2380. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

