Dealing with Danger in the CNS: The Response of the Immune System to Injury
- PMID: 26139369
- PMCID: PMC4491143
- DOI: 10.1016/j.neuron.2015.05.019
Dealing with Danger in the CNS: The Response of the Immune System to Injury
Abstract
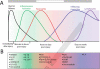
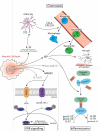
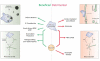
Fighting pathogens and maintaining tissue homeostasis are prerequisites for survival. Both of these functions are upheld by the immune system, though the latter is often overlooked in the context of the CNS. The mere presence of immune cells in the CNS was long considered a hallmark of pathology, but this view has been recently challenged by studies demonstrating that immunological signaling can confer pivotal neuroprotective effects on the injured CNS. In this review, we describe the temporal sequence of immunological events that follow CNS injury. Beginning with immediate changes at the injury site, including death of neural cells and release of damage-associated molecular patterns (DAMPs), and progressing through innate and adaptive immune responses, we describe the cascade of inflammatory mediators and the implications of their post-injury effects. We conclude by proposing a revised interpretation of immune privilege in the brain, which takes beneficial neuro-immune communications into account.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Restoring neuro-immune circuitry after brain and spinal cord injuries.Int Immunol. 2021 Jun 7;33(6):311-325. doi: 10.1093/intimm/dxab017. Int Immunol. 2021. PMID: 33851981 Review.
-
Complement activation in the injured central nervous system: another dual-edged sword?J Neuroinflammation. 2012 Jun 21;9:137. doi: 10.1186/1742-2094-9-137. J Neuroinflammation. 2012. PMID: 22721265 Free PMC article. Review.
-
The systemic response to CNS injury.Exp Neurol. 2014 Aug;258:105-11. doi: 10.1016/j.expneurol.2014.03.013. Exp Neurol. 2014. PMID: 25017891 Review.
-
Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury.J Cereb Blood Flow Metab. 2014 Mar;34(3):369-75. doi: 10.1038/jcbfm.2013.227. Epub 2014 Jan 8. J Cereb Blood Flow Metab. 2014. PMID: 24398940 Free PMC article. Review.
-
Effects of 9-t-butyl doxycycline on the innate immune response to CNS ischemia-reperfusion injury.Exp Mol Pathol. 2021 Feb;118:104601. doi: 10.1016/j.yexmp.2020.104601. Epub 2020 Dec 29. Exp Mol Pathol. 2021. PMID: 33385413 Free PMC article.
Cited by
-
Advancements and mechanisms of stem cell-based therapies for spinal cord injury in animals.Int J Surg. 2024 Oct 1;110(10):6182-6197. doi: 10.1097/JS9.0000000000001074. Int J Surg. 2024. PMID: 38265419 Free PMC article. Review.
-
Roadmap for Stroke: Challenging the Role of the Neuronal Extracellular Matrix.Int J Mol Sci. 2020 Oct 13;21(20):7554. doi: 10.3390/ijms21207554. Int J Mol Sci. 2020. PMID: 33066304 Free PMC article. Review.
-
A novel injury paradigm in the central nervous system of adult Drosophila: molecular, cellular and functional aspects.Dis Model Mech. 2021 May 1;14(5):dmm044669. doi: 10.1242/dmm.044669. Epub 2021 Jun 1. Dis Model Mech. 2021. PMID: 34061177 Free PMC article.
-
Cathepsins in neuronal plasticity.Neural Regen Res. 2021 Jan;16(1):26-35. doi: 10.4103/1673-5374.286948. Neural Regen Res. 2021. PMID: 32788444 Free PMC article.
-
Stem Cell Therapy for Modulating Neuroinflammation in Neuropathic Pain.Int J Mol Sci. 2021 May 3;22(9):4853. doi: 10.3390/ijms22094853. Int J Mol Sci. 2021. PMID: 34063721 Free PMC article. Review.
References
-
- Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annual review of immunology. 2012;30:459–489. - PubMed
-
- Archambault AS, Sim J, Gimenez MA, Russell JH. Defining antigen-dependent stages of T cell migration from the blood to the central nervous system parenchyma. Eur J Immunol. 2005;35:1076–1085. - PubMed
-
- Bareyre F, Wahl F, McIntosh TK, Stutzmann JM. Time course of cerebral edema after traumatic brain injury in rats: effects of riluzole and mannitol. Journal of neurotrauma. 1997;14:839–849. - PubMed
-
- Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:1708–1716. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

