Suppression of experimental arthritis through AMP-activated protein kinase activation and autophagy modulation
- PMID: 26120598
- PMCID: PMC4479345
- DOI: 10.23937/2469-5726/1510005
Suppression of experimental arthritis through AMP-activated protein kinase activation and autophagy modulation
Abstract
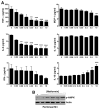
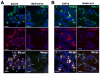
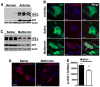
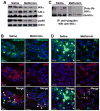
Autophagy plays a central role in various disease processes. However, its contribution to inflammatory arthritides such as rheumatoid arthritis (RA) is unclear. We observed that autophagy is engaged in the K/BxN serum transfer model of RA but autophagic flux is severely impaired. Metformin is an anti-diabetic drug that has been shown to stimulate autophagy. Induction of autophagic flux, through metformin-mediated AMP-activated protein kinase (AMPK) activation and interruption of mammalian target of rapamycin (mTOR) signaling mitigated the inflammation in experimental arthritis. Further investigation into the effects of metformin suggest that the drug directly activates AMPK and dose-dependently suppressed the release of TNF-α, IL-6, and MCP-1 by macrophages while enhancing the release of IL-10 in vitro. In vivo, metformin treatment significantly suppressed clinical arthritis and inflammatory cytokine production. Mechanistic studies suggest that metformin exerts its anti-inflammatory effects by correcting the impaired autophagic flux observed in the K/BxN arthritis model and suppressing NF-κB-mediated signaling through selective degradation of IκB kinase (IKK). These findings establish a central role for autophagy in inflammatory arthritis and argue that autophagy modulators such as metformin may represent potential therapeutic agents for the treatment of RA.
Keywords: AMP-activated protein kinase (AMPK); Autophagy; inflammatory arthritis; mammalian target of rapamycin (mTOR); metformin.
Conflict of interest statement
Conflict: The authors declare no conflict of interest
Figures


Similar articles
-
Fumagillin prodrug nanotherapy suppresses macrophage inflammatory response via endothelial nitric oxide.ACS Nano. 2014 Jul 22;8(7):7305-17. doi: 10.1021/nn502372n. Epub 2014 Jun 24. ACS Nano. 2014. PMID: 24941020 Free PMC article.
-
Metformin inhibits TNF-alpha-induced IkappaB kinase phosphorylation, IkappaB-alpha degradation and IL-6 production in endothelial cells through PI3K-dependent AMPK phosphorylation.Int J Cardiol. 2009 May 15;134(2):169-75. doi: 10.1016/j.ijcard.2008.04.010. Epub 2008 Jul 1. Int J Cardiol. 2009. PMID: 18597869
-
Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells.Hypertension. 2006 Jun;47(6):1183-8. doi: 10.1161/01.HYP.0000221429.94591.72. Epub 2006 Apr 24. Hypertension. 2006. PMID: 16636195
-
Regulation of autophagy by stress-responsive transcription factors.Semin Cancer Biol. 2013 Oct;23(5):310-22. doi: 10.1016/j.semcancer.2013.05.008. Epub 2013 May 30. Semin Cancer Biol. 2013. PMID: 23726895 Review.
-
Metformin one in a Million Efficient Medicines for Rheumatoid Arthritis Complications: Inflammation, Osteoblastogenesis, Cardiovascular Disease, Malignancies.Curr Rheumatol Rev. 2019;15(2):116-122. doi: 10.2174/1573397114666180717145745. Curr Rheumatol Rev. 2019. PMID: 30019648 Review.
Cited by
-
Immunomodulatory Effects of SGLT2 Inhibitors and Metformin in Managing Rheumatic Diseases: A Narrative Review.Mediterr J Rheumatol. 2024 Sep 30;35(3):411-421. doi: 10.31138/mjr.010324.ies. eCollection 2024 Sep. Mediterr J Rheumatol. 2024. PMID: 39463877 Free PMC article. Review.
-
Mechanistic target of rapamycin (mTOR): a potential new therapeutic target for rheumatoid arthritis.Arthritis Res Ther. 2023 Oct 2;25(1):187. doi: 10.1186/s13075-023-03181-w. Arthritis Res Ther. 2023. PMID: 37784141 Free PMC article. Review.
-
[Energy metabolism of the immune system : Consequences in chronic inflammation].Z Rheumatol. 2023 Aug;82(6):479-490. doi: 10.1007/s00393-023-01389-4. Epub 2023 Jul 24. Z Rheumatol. 2023. PMID: 37488246 Review. German.
-
Interactions of Autophagy and the Immune System in Health and Diseases.Autophagy Rep. 2022;1(1):438-515. doi: 10.1080/27694127.2022.2119743. Epub 2022 Oct 5. Autophagy Rep. 2022. PMID: 37425656 Free PMC article.
-
Metformin attenuates symptoms of osteoarthritis: role of genetic diversity of Bcl2 and CXCL16 in OA.Arthritis Res Ther. 2023 Mar 7;25(1):35. doi: 10.1186/s13075-023-03025-7. Arthritis Res Ther. 2023. PMID: 36879307 Free PMC article. Clinical Trial.
References
-
- McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–42. - PubMed
-
- Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–99. - PubMed
-
- Munz C. Enhancing immunity through autophagy. Annu Rev Immunol. 2009;27:423–49. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
