Genetically induced moderate inhibition of 20S proteasomes in cardiomyocytes facilitates heart failure in mice during systolic overload
- PMID: 26116868
- PMCID: PMC4530032
- DOI: 10.1016/j.yjmcc.2015.06.014
Genetically induced moderate inhibition of 20S proteasomes in cardiomyocytes facilitates heart failure in mice during systolic overload
Abstract
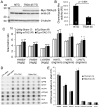
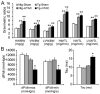
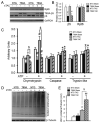
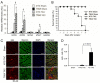
The in vivo function status of the ubiquitin-proteasome system (UPS) in pressure overloaded hearts remains undefined. Cardiotoxicity was observed during proteasome inhibitor chemotherapy, especially in those with preexisting cardiovascular conditions; however, proteasome inhibition (PsmI) was also suggested by some experimental studies as a potential therapeutic strategy to curtail cardiac hypertrophy. Here we used genetic approaches to probe cardiac UPS performance and determine the impact of cardiomyocyte-restricted PsmI (CR-PsmI) on cardiac responses to systolic overload. Transgenic mice expressing an inverse reporter of the UPS (GFPdgn) were subject to transverse aortic constriction (TAC) to probe myocardial UPS performance during systolic overload. Mice with or without moderate CR-PsmI were subject to TAC and temporally characterized for cardiac responses to moderate and severe systolic overload. After moderate TAC (pressure gradient: ~40mmHg), cardiac UPS function was upregulated during the first two weeks but turned to functional insufficiency between 6 and 12weeks as evidenced by the dynamic changes in GFPdgn protein levels, proteasome peptidase activities, and total ubiquitin conjugates. Severe TAC (pressure gradients >60mmHg) led to UPS functional insufficiency within a week. Moderate TAC elicited comparable hypertrophic responses between mice with and without genetic CR-PsmI but caused cardiac malfunction in CR-PsmI mice significantly earlier than those without CR-PsmI. In mice subject to severe TAC, CR-PsmI inhibited cardiac hypertrophy but led to rapidly progressed heart failure and premature death, associated with a pronounced increase in cardiomyocyte death. It is concluded that cardiac UPS function is dynamically altered, with the initial brief upregulation of proteasome function being adaptive; and CR-PsmI facilitates cardiac malfunction during systolic overload.
Keywords: Cardiac hypertrophy; Heart failure; Pressure overload; Proteasome inhibition; Transgenic mice; Ubiquitin–proteasome system.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Dynamic regulation of the proteasome by systolic overload.J Mol Cell Cardiol. 2015 Oct;87:1-3. doi: 10.1016/j.yjmcc.2015.07.011. Epub 2015 Jul 26. J Mol Cell Cardiol. 2015. PMID: 26219953 No abstract available.
Similar articles
-
Cardiac proteasome functional insufficiency plays a pathogenic role in diabetic cardiomyopathy.J Mol Cell Cardiol. 2017 Jan;102:53-60. doi: 10.1016/j.yjmcc.2016.11.013. Epub 2016 Nov 30. J Mol Cell Cardiol. 2017. PMID: 27913284 Free PMC article.
-
Genetically induced moderate inhibition of the proteasome in cardiomyocytes exacerbates myocardial ischemia-reperfusion injury in mice.Circ Res. 2012 Aug 17;111(5):532-42. doi: 10.1161/CIRCRESAHA.112.270983. Epub 2012 Jun 26. Circ Res. 2012. PMID: 22740087 Free PMC article.
-
The Calcineurin-TFEB-p62 Pathway Mediates the Activation of Cardiac Macroautophagy by Proteasomal Malfunction.Circ Res. 2020 Jul 31;127(4):502-518. doi: 10.1161/CIRCRESAHA.119.316007. Epub 2020 May 5. Circ Res. 2020. PMID: 32366200 Free PMC article.
-
Perturbation of cullin deneddylation via conditional Csn8 ablation impairs the ubiquitin-proteasome system and causes cardiomyocyte necrosis and dilated cardiomyopathy in mice.Circ Res. 2011 Jan 7;108(1):40-50. doi: 10.1161/CIRCRESAHA.110.230607. Epub 2010 Nov 4. Circ Res. 2011. PMID: 21051661 Free PMC article.
-
Inadequate ubiquitination-proteasome coupling contributes to myocardial ischemia-reperfusion injury.J Clin Invest. 2018 Dec 3;128(12):5294-5306. doi: 10.1172/JCI98287. Epub 2018 Oct 22. J Clin Invest. 2018. PMID: 30204128 Free PMC article.
Cited by
-
Mitochondrial Quality Control and Cellular Proteostasis: Two Sides of the Same Coin.Front Physiol. 2020 May 25;11:515. doi: 10.3389/fphys.2020.00515. eCollection 2020. Front Physiol. 2020. PMID: 32528313 Free PMC article. Review.
-
The double-hit protocol induces HFpEF and impairs myocardial ubiquitin-proteasome system performance in FVB/N mice.Front Physiol. 2023 Jun 8;14:1208153. doi: 10.3389/fphys.2023.1208153. eCollection 2023. Front Physiol. 2023. PMID: 37362441 Free PMC article.
-
PDE1 inhibition facilitates proteasomal degradation of misfolded proteins and protects against cardiac proteinopathy.Sci Adv. 2019 May 22;5(5):eaaw5870. doi: 10.1126/sciadv.aaw5870. eCollection 2019 May. Sci Adv. 2019. PMID: 31131329 Free PMC article.
-
Cardiac proteasome functional insufficiency plays a pathogenic role in diabetic cardiomyopathy.J Mol Cell Cardiol. 2017 Jan;102:53-60. doi: 10.1016/j.yjmcc.2016.11.013. Epub 2016 Nov 30. J Mol Cell Cardiol. 2017. PMID: 27913284 Free PMC article.
-
Mechanisms shared between cancer, heart failure, and targeted anti-cancer therapies.Cardiovasc Res. 2023 Feb 3;118(18):3451-3466. doi: 10.1093/cvr/cvac132. Cardiovasc Res. 2023. PMID: 36004495 Free PMC article. Review.
References
-
- Schlossarek S, Frey N, Carrier L. Ubiquitin-proteasome system and hereditary cardiomyopathies. J Mol Cell Cardiol. 2014;71:25–31. - PubMed
-
- Tsukamoto O, Minamino T, Kitakaze M. Functional alterations of cardiac proteasomes under physiological and pathological conditions. Cardiovasc Res. 2009;14:14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

