Recombinant Immunomodulating Lentogenic or Mesogenic Oncolytic Newcastle Disease Virus for Treatment of Pancreatic Adenocarcinoma
- PMID: 26110582
- PMCID: PMC4488723
- DOI: 10.3390/v7062756
Recombinant Immunomodulating Lentogenic or Mesogenic Oncolytic Newcastle Disease Virus for Treatment of Pancreatic Adenocarcinoma
Abstract
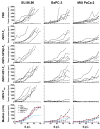
Oncolytic Newcastle disease virus (NDV) might be a promising new therapeutic agent for the treatment of pancreatic cancer. We evaluated recombinant NDVs (rNDVs) expressing interferon (rNDV-hIFNβ-F₀) or an IFN antagonistic protein (rNDV-NS1-F₀), as well as rNDV with increased virulence (rNDV-F₃aa) for oncolytic efficacy in human pancreatic adenocarcinoma cells. Expression of additional proteins did not hamper virus replication or cytotoxic effects on itself. However, expression of interferon, but not NS1, resulted in loss of multicycle replication. Conversely, increasing the virulence (rNDV-F₃aa) resulted in enhanced replication of the virus. Type I interferon was produced in high amounts by all tumor cells inoculated with rNDV-hIFNβ -F₀, while inoculation with rNDV-NS1-F₀ resulted in a complete block of interferon production in most cells. Inoculation of human pancreatic adenocarcinoma cells with rNDV-F₃aa caused markedly more cytotoxicity compared to rNDV-F₀, while inoculation with rNDVβ-hIFN -F₀ and rNDV-NS1-F₀ induced cytotoxic effects comparable to those induced by the parental rNDV-F₀. Evaluation in vivo using mice bearing subcutaneous pancreatic cancer xenografts revealed that only intratumoral injection with rNDV-F₃aa resulted in regression of tumors. We conclude that although lentogenic rNDVs harboring proteins that modulate the type I interferon pathway proteins do have an oncolytic effect, a more virulent mesogenic rNDV might be needed to improve oncolytic efficacy.
Keywords: Newcastle disease virus; immunotherapy; innate immunity; oncolytic virotherapy; oncolytic virus; pancreatic adenocarcinoma.
Figures
Similar articles
-
Type I interferon-sensitive recombinant newcastle disease virus for oncolytic virotherapy.J Virol. 2010 Apr;84(8):3835-44. doi: 10.1128/JVI.01553-09. Epub 2010 Feb 10. J Virol. 2010. PMID: 20147405 Free PMC article.
-
Engineering Newcastle Disease Virus as an Oncolytic Vector for Intratumoral Delivery of Immune Checkpoint Inhibitors and Immunocytokines.J Virol. 2020 Jan 17;94(3):e01677-19. doi: 10.1128/JVI.01677-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31694938 Free PMC article.
-
Oncolytic Newcastle disease virus expressing chimeric antibody enhanced anti-tumor efficacy in orthotopic hepatoma-bearing mice.J Exp Clin Cancer Res. 2015 Dec 21;34:153. doi: 10.1186/s13046-015-0271-1. J Exp Clin Cancer Res. 2015. PMID: 26689432 Free PMC article.
-
[Expressing foreign genes by Newcastle disease virus for cancer therapy].Mol Biol (Mosk). 2015 Mar-Apr;49(2):195-204. doi: 10.7868/s0026898415020020. Mol Biol (Mosk). 2015. PMID: 26065249 Review. Russian.
-
Oncolytic therapy and gene therapy for cancer: recent advances in antitumor effects of Newcastle disease virus.Discov Med. 2020 Jul-Aug;30(159):39-48. Discov Med. 2020. PMID: 33357361 Review.
Cited by
-
Microorganisms in chemotherapy for pancreatic cancer: An overview of current research and future directions.Int J Biol Sci. 2021 Jun 26;17(10):2666-2682. doi: 10.7150/ijbs.59117. eCollection 2021. Int J Biol Sci. 2021. PMID: 34326701 Free PMC article. Review.
-
Current strategies in engaging oncolytic viruses with antitumor immunity.Mol Ther Oncolytics. 2021 May 14;22:98-113. doi: 10.1016/j.omto.2021.05.002. eCollection 2021 Sep 24. Mol Ther Oncolytics. 2021. PMID: 34514092 Free PMC article. Review.
-
An Extensive Review on Preclinical and Clinical Trials of Oncolytic Viruses Therapy for Pancreatic Cancer.Front Oncol. 2022 May 24;12:875188. doi: 10.3389/fonc.2022.875188. eCollection 2022. Front Oncol. 2022. PMID: 35686109 Free PMC article. Review.
-
Armed oncolytic viruses: A kick-start for anti-tumor immunity.Cytokine Growth Factor Rev. 2018 Jun;41:28-39. doi: 10.1016/j.cytogfr.2018.03.006. Epub 2018 Mar 19. Cytokine Growth Factor Rev. 2018. PMID: 29576283 Free PMC article. Review.
-
Oncolytic effect of wild-type Newcastle disease virus isolates in cancer cell lines in vitro and in vivo on xenograft model.PLoS One. 2018 Apr 5;13(4):e0195425. doi: 10.1371/journal.pone.0195425. eCollection 2018. PLoS One. 2018. PMID: 29621357 Free PMC article.
References
-
- Neoptolemos J.P., Stocken D.D., Bassi C., Ghaneh P., Cunningham D., Goldstein D., Padbury R., Moore M.J., Gallinger S., Mariette C., et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: A randomized controlled trial. JAMA. 2010;304:1073–1081. doi: 10.1001/jama.2010.1275. - DOI - PubMed
-
- Prince A.M., Ginsberg H.S. Studies on the cytotoxic effect of newcastle disease virus (NDV) on ehrlich ascites tumor cells. Ii. The mechanism and significance of in vitro recovery from the effect of ndv. J. Immunol. 1957;79:107–112. - PubMed
-
- Prince A.M., Ginsberg H.S. Studies on the cytotoxic effect of newcastle disease virus (NDV) on ehrlich ascites tumor cells. I. Characteristics of the virus-cell interaction. J. Immunol. 1957;79:94–106. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

