Identification of a New Class of Antifungals Targeting the Synthesis of Fungal Sphingolipids
- PMID: 26106079
- PMCID: PMC4479701
- DOI: 10.1128/mBio.00647-15
Identification of a New Class of Antifungals Targeting the Synthesis of Fungal Sphingolipids
Erratum in
-
Erratum for Mor et al., "Identification of a New Class of Antifungals Targeting the Synthesis of Fungal Sphingolipids".mBio. 2018 Mar 13;9(2):e00188-18. doi: 10.1128/mBio.00188-18. mBio. 2018. PMID: 29535196 Free PMC article. No abstract available.
Abstract
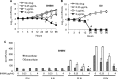
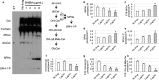
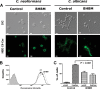
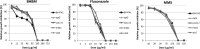
Recent estimates suggest that >300 million people are afflicted by serious fungal infections worldwide. Current antifungal drugs are static and toxic and/or have a narrow spectrum of activity. Thus, there is an urgent need for the development of new antifungal drugs. The fungal sphingolipid glucosylceramide (GlcCer) is critical in promoting virulence of a variety of human-pathogenic fungi. In this study, we screened a synthetic drug library for compounds that target the synthesis of fungal, but not mammalian, GlcCer and found two compounds [N'-(3-bromo-4-hydroxybenzylidene)-2-methylbenzohydrazide (BHBM) and its derivative, 3-bromo-N'-(3-bromo-4-hydroxybenzylidene) benzohydrazide (D0)] that were highly effective in vitro and in vivo against several pathogenic fungi. BHBM and D0 were well tolerated in animals and are highly synergistic or additive to current antifungals. BHBM and D0 significantly affected fungal cell morphology and resulted in the accumulation of intracellular vesicles. Deep-sequencing analysis of drug-resistant mutants revealed that four protein products, encoded by genes APL5, COS111, MKK1, and STE2, which are involved in vesicular transport and cell cycle progression, are targeted by BHBM.
Importance: Fungal infections are a significant cause of morbidity and mortality worldwide. Current antifungal drugs suffer from various drawbacks, including toxicity, drug resistance, and narrow spectrum of activity. In this study, we have demonstrated that pharmaceutical inhibition of fungal glucosylceramide presents a new opportunity to treat cryptococcosis and various other fungal infections. In addition to being effective against pathogenic fungi, the compounds discovered in this study were well tolerated by animals and additive to current antifungals. These findings suggest that these drugs might pave the way for the development of a new class of antifungals.
Copyright © 2015 Mor et al.
Figures



Similar articles
-
Sphingolipids as targets for treatment of fungal infections.Future Med Chem. 2016 Aug;8(12):1469-84. doi: 10.4155/fmc-2016-0053. Epub 2016 Aug 9. Future Med Chem. 2016. PMID: 27502288 Free PMC article. Review.
-
Acylhydrazones as Antifungal Agents Targeting the Synthesis of Fungal Sphingolipids.Antimicrob Agents Chemother. 2018 Apr 26;62(5):e00156-18. doi: 10.1128/AAC.00156-18. Print 2018 May. Antimicrob Agents Chemother. 2018. PMID: 29507066 Free PMC article.
-
Preclinical Evaluation of Acylhydrazone SB-AF-1002 as a Novel Broad-Spectrum Antifungal Agent.Antimicrob Agents Chemother. 2020 Aug 20;64(9):e00946-20. doi: 10.1128/AAC.00946-20. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32601165 Free PMC article.
-
Fungal sphingolipids: role in the regulation of virulence and potential as targets for future antifungal therapies.Expert Rev Anti Infect Ther. 2020 Nov;18(11):1083-1092. doi: 10.1080/14787210.2020.1792288. Epub 2020 Jul 16. Expert Rev Anti Infect Ther. 2020. PMID: 32673125 Free PMC article. Review.
-
SAR Studies on Aromatic Acylhydrazone-Based Inhibitors of Fungal Sphingolipid Synthesis as Next-Generation Antifungal Agents.J Med Chem. 2019 Sep 12;62(17):8249-8273. doi: 10.1021/acs.jmedchem.9b01004. Epub 2019 Aug 16. J Med Chem. 2019. PMID: 31369263 Free PMC article.
Cited by
-
Deciphering the Role of PIG1 and DHN-Melanin in Scedosporium apiospermum Conidia.J Fungi (Basel). 2023 Jan 18;9(2):134. doi: 10.3390/jof9020134. J Fungi (Basel). 2023. PMID: 36836250 Free PMC article.
-
Monoclonal Antibodies against Cell Wall Chitooligomers as Accessory Tools for the Control of Cryptococcosis.Antimicrob Agents Chemother. 2021 Nov 17;65(12):e0118121. doi: 10.1128/AAC.01181-21. Epub 2021 Sep 27. Antimicrob Agents Chemother. 2021. PMID: 34570650 Free PMC article.
-
Sphingolipids as targets for treatment of fungal infections.Future Med Chem. 2016 Aug;8(12):1469-84. doi: 10.4155/fmc-2016-0053. Epub 2016 Aug 9. Future Med Chem. 2016. PMID: 27502288 Free PMC article. Review.
-
Inositol Phosphoryl Transferase, Ipt1, Is a Critical Determinant of Azole Resistance and Virulence Phenotypes in Candida glabrata.J Fungi (Basel). 2022 Jun 21;8(7):651. doi: 10.3390/jof8070651. J Fungi (Basel). 2022. PMID: 35887407 Free PMC article.
-
New Approaches for Cryptococcosis Treatment.Microorganisms. 2020 Apr 23;8(4):613. doi: 10.3390/microorganisms8040613. Microorganisms. 2020. PMID: 32340403 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI071142/AI/NIAID NIH HHS/United States
- AI71142/AI/NIAID NIH HHS/United States
- T32 CA121940/CA/NCI NIH HHS/United States
- R56 AI087541/AI/NIAID NIH HHS/United States
- AI87541/AI/NIAID NIH HHS/United States
- HHSN27200002/A51/PHS HHS/United States
- R01 AI047837/AI/NIAID NIH HHS/United States
- R01 AI056168/AI/NIAID NIH HHS/United States
- AI56168/AI/NIAID NIH HHS/United States
- HHSN272201000029I/PHS HHS/United States
- R21 AI100631/AI/NIAID NIH HHS/United States
- HG004840/HG/NHGRI NIH HHS/United States
- R01 HG004840/HG/NHGRI NIH HHS/United States
- R56 AI047837/AI/NIAID NIH HHS/United States
- AI100631/AI/NIAID NIH HHS/United States
- T32 AI007539/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
