Pharmacokinetics and Toxicity of Sodium Selenite in the Treatment of Patients with Carcinoma in a Phase I Clinical Trial: The SECAR Study
- PMID: 26102212
- PMCID: PMC4488827
- DOI: 10.3390/nu7064978
Pharmacokinetics and Toxicity of Sodium Selenite in the Treatment of Patients with Carcinoma in a Phase I Clinical Trial: The SECAR Study
Abstract
Background: Sodium selenite at high dose exerts antitumor effects and increases efficacy of cytostatic drugs in multiple preclinical malignancy models. We assessed the safety and efficacy of intravenous administered sodium selenite in cancer patients' refractory to cytostatic drugs in a phase I trial. Patients received first line of chemotherapy following selenite treatment to investigate altered sensitivity to these drugs and preliminary assessment of any clinical benefits.
Materials and methods: Thirty-four patients with different therapy resistant tumors received iv sodium selenite daily for consecutive five days either for two weeks or four weeks. Each cohort consisted of at least three patients who received the same daily dose of selenite throughout the whole treatment. If 0/3 patients had dose-limiting toxicities (DLTs), the study proceeded to the next dose-level. If 2/3 had DLT, the dose was considered too high and if 1/3 had DLT, three more patients were included. Dose-escalation continued until the maximum tolerated dose (MTD) was reached. MTD was defined as the highest dose-level on which 0/3 or 1/6 patients experienced DLT. The primary endpoint was safety, dose-limiting toxic effects and the MTD of sodium selenite. The secondary endpoint was primary response evaluation.
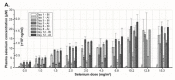
Results and conclusion: MTD was defined as 10.2 mg/m(2), with a calculated median plasma half-life of 18.25 h. The maximum plasma concentration of selenium from a single dose of selenite increased in a nonlinear pattern. The most common adverse events were fatigue, nausea, and cramps in fingers and legs. DLTs were acute, of short duration and reversible. Biomarkers for organ functions indicated no major systemic toxicity. In conclusion, sodium selenite is safe and tolerable when administered up to 10.2 mg/m(2) under current protocol. Further development of the study is underway to determine if prolonged infusions might be a more effective treatment strategy.
Keywords: carcinoma; maximum tolerated dose; pharmacokinetics; sodium selenite.
Figures



Similar articles
-
Phase I clinical trial and pharmacokinetic study of the spicamycin analog KRN5500 administered as a 1-hour intravenous infusion for five consecutive days to patients with refractory solid tumors.Clin Cancer Res. 2003 Nov 1;9(14):5178-86. Clin Cancer Res. 2003. PMID: 14613997 Clinical Trial.
-
Phase I and pharmacokinetic study of E7070, a chloroindolyl-sulfonamide anticancer agent, administered on a weekly schedule to patients with solid tumors.Clin Cancer Res. 2003 Nov 1;9(14):5195-204. Clin Cancer Res. 2003. PMID: 14613999 Clinical Trial.
-
Phase I and pharmacokinetic study of a daily times 5 short intravenous infusion schedule of 9-aminocamptothecin in a colloidal dispersion formulation in patients with advanced solid tumors.J Clin Oncol. 1999 Jun;17(6):1906-14. doi: 10.1200/JCO.1999.17.6.1906. J Clin Oncol. 1999. PMID: 10561232 Clinical Trial.
-
Trends in the characteristics, dose-limiting toxicities and efficacy of phase I oncology trials: The Cancer Research UK experience.Eur J Cancer. 2016 Oct;66:9-16. doi: 10.1016/j.ejca.2016.07.004. Epub 2016 Aug 8. Eur J Cancer. 2016. PMID: 27514008 Review.
-
Concept of maximum tolerated systemic exposure and its application to phase I-II studies of anticancer drugs.Med Pediatr Oncol. 1991;19(3):153-9. doi: 10.1002/mpo.2950190302. Med Pediatr Oncol. 1991. PMID: 2023562 Review.
Cited by
-
Selenoprotein P as Biomarker of Selenium Status in Clinical Trials with Therapeutic Dosages of Selenite.Nutrients. 2020 Apr 12;12(4):1067. doi: 10.3390/nu12041067. Nutrients. 2020. PMID: 32290626 Free PMC article. Clinical Trial.
-
Selenium Deficiency Due to Diet, Pregnancy, Severe Illness, or COVID-19-A Preventable Trigger for Autoimmune Disease.Int J Mol Sci. 2021 Aug 8;22(16):8532. doi: 10.3390/ijms22168532. Int J Mol Sci. 2021. PMID: 34445238 Free PMC article. Review.
-
Dietary Selenium and Human Health.Nutrients. 2016 Dec 30;9(1):22. doi: 10.3390/nu9010022. Nutrients. 2016. PMID: 28042811 Free PMC article.
-
Potential Benefits of Selenium Supplementation in Reducing Insulin Resistance in Patients with Cardiometabolic Diseases: A Systematic Review and Meta-Analysis.Nutrients. 2022 Nov 21;14(22):4933. doi: 10.3390/nu14224933. Nutrients. 2022. PMID: 36432623 Free PMC article. Review.
-
Effect of Selenium on the Iron Homeostasis and Oxidative Damage in Brain and Liver of Mice.Antioxidants (Basel). 2022 Jun 21;11(7):1216. doi: 10.3390/antiox11071216. Antioxidants (Basel). 2022. PMID: 35883707 Free PMC article.
References
-
- Yan L., Spallholz J.E. Generation of reactive oxygen species from the reaction of selenium compounds with thiols and mammary tumor cells. Biochem. Pharmacol. 1993;45:429–437. - PubMed
-
- Jiang Q., Wang Y., Li T., Shi K., Li Z., Ma Y., Li F., Luo H., Yang Y., Xu C. Heat shock protein 90–mediated inactivation of nuclear factor-κb switches autophagy to apoptosis through becn1 transcriptional inhibition in selenite-induced nb4 cells. Mol. Biol. Cell. 2011;22:1167–1180. doi: 10.1091/mbc.E10-10-0860. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

