Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice
- PMID: 26100496
- PMCID: PMC4615393
- DOI: 10.1016/j.jhep.2015.06.013
Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice
Abstract
Background & aims: The inflammasome is a well-characterized inducer of inflammation in alcoholic steatohepatitis (ASH). Inflammasome activation requires two signals for mature interleukin (IL)-1β production. Here we asked whether metabolic danger signals trigger inflammasome activation in ASH.
Methods: Wild-type mice, ATP receptor 2x7 (P2rx7)-KO mice, or mice overexpressing uricase were fed Lieber-DeCarli ethanol or control diet. We also implemented a pharmacological approach in which mice were treated with probenecid or allopurinol.
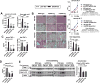
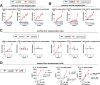
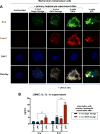
Results: The sterile danger signals, ATP and uric acid, were increased in the serum and liver of alcohol-fed mice. Depletion of uric acid or ATP, or lack of ATP signaling attenuated ASH and prevented inflammasome activation and its major downstream cytokine, IL-1β. Pharmacological depletion of uric acid with allopurinol provided significant protection from alcohol-induced inflammatory response, steatosis and liver damage, and additional protection was achieved in mice treated with probenecid, which depletes uric acid and blocks ATP-induced P2rx7 signaling. We found that alcohol-damaged hepatocytes released uric acid and ATP in vivo and in vitro and that these sterile danger signals activated the inflammasome in LPS-exposed liver mononuclear cells.
Conclusions: Our data indicate that the second signal in inflammasome activation and IL-1β production in ASH results from the endogenous danger signals, uric acid and ATP. Inhibition of signaling triggered by uric acid and ATP may have therapeutic implications in ASH.
Keywords: Alcoholic steatohepatitis; Damage-associated molecular patterns; Determinants of liver inflammation; Inflammasome; Pathogen-associated molecular patterns; Sterile inflammatory response.
Copyright © 2015 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures
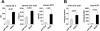


Similar articles
-
Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease.J Leukoc Biol. 2015 Aug;98(2):249-56. doi: 10.1189/jlb.3AB1214-590R. Epub 2015 May 1. J Leukoc Biol. 2015. PMID: 25934928 Free PMC article.
-
Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells.Hepatology. 2011 Jul;54(1):133-44. doi: 10.1002/hep.24341. Hepatology. 2011. PMID: 21488066 Free PMC article.
-
Progression of non-alcoholic steatosis to steatohepatitis and fibrosis parallels cumulative accumulation of danger signals that promote inflammation and liver tumors in a high fat-cholesterol-sugar diet model in mice.J Transl Med. 2015 Jun 16;13:193. doi: 10.1186/s12967-015-0552-7. J Transl Med. 2015. PMID: 26077675 Free PMC article.
-
IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice.J Clin Invest. 2012 Oct;122(10):3476-89. doi: 10.1172/JCI60777. Epub 2012 Sep 4. J Clin Invest. 2012. PMID: 22945633 Free PMC article. Review.
-
Sterile inflammation in the liver.Gastroenterology. 2012 Nov;143(5):1158-1172. doi: 10.1053/j.gastro.2012.09.008. Epub 2012 Sep 13. Gastroenterology. 2012. PMID: 22982943 Review.
Cited by
-
Alcohol, Inflammation, and Microbiota in Alcoholic Liver Disease.Int J Mol Sci. 2023 Feb 13;24(4):3735. doi: 10.3390/ijms24043735. Int J Mol Sci. 2023. PMID: 36835145 Free PMC article. Review.
-
Alcohol dysregulates miR-148a in hepatocytes through FoxO1, facilitating pyroptosis via TXNIP overexpression.Gut. 2019 Apr;68(4):708-720. doi: 10.1136/gutjnl-2017-315123. Epub 2018 Feb 23. Gut. 2019. PMID: 29475852 Free PMC article.
-
Neutrophil extracellular traps activate hepatic stellate cells and monocytes via NLRP3 sensing in alcohol-induced acceleration of MASH fibrosis.Gut. 2024 Oct 7;73(11):1854-1869. doi: 10.1136/gutjnl-2023-331447. Gut. 2024. PMID: 38777573 Free PMC article.
-
Alcohol abuse is associated with enhanced pulmonary and systemic xanthine oxidoreductase activity.Am J Physiol Lung Cell Mol Physiol. 2017 Dec 1;313(6):L1047-L1057. doi: 10.1152/ajplung.00570.2016. Epub 2017 Aug 24. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28839105 Free PMC article.
-
Recent Advances in Understanding of Pathogenesis of Alcohol-Associated Liver Disease.Annu Rev Pathol. 2023 Jan 24;18:411-438. doi: 10.1146/annurev-pathmechdis-031521-030435. Epub 2022 Oct 21. Annu Rev Pathol. 2023. PMID: 36270295 Free PMC article. Review.
References
-
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

