Artery Tertiary Lymphoid Organs Control Aorta Immunity and Protect against Atherosclerosis via Vascular Smooth Muscle Cell Lymphotoxin β Receptors
- PMID: 26084025
- PMCID: PMC4678289
- DOI: 10.1016/j.immuni.2015.05.015
Artery Tertiary Lymphoid Organs Control Aorta Immunity and Protect against Atherosclerosis via Vascular Smooth Muscle Cell Lymphotoxin β Receptors
Abstract
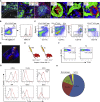
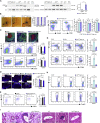
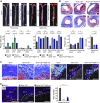
Tertiary lymphoid organs (TLOs) emerge during nonresolving peripheral inflammation, but their impact on disease progression remains unknown. We have found in aged Apoe(-/-) mice that artery TLOs (ATLOs) controlled highly territorialized aorta T cell responses. ATLOs promoted T cell recruitment, primed CD4(+) T cells, generated CD4(+), CD8(+), T regulatory (Treg) effector and central memory cells, converted naive CD4(+) T cells into induced Treg cells, and presented antigen by an unusual set of dendritic cells and B cells. Meanwhile, vascular smooth muscle cell lymphotoxin β receptors (VSMC-LTβRs) protected against atherosclerosis by maintaining structure, cellularity, and size of ATLOs though VSMC-LTβRs did not affect secondary lymphoid organs: Atherosclerosis was markedly exacerbated in Apoe(-/-)Ltbr(-/-) and to a similar extent in aged Apoe(-/-)Ltbr(fl/fl)Tagln-cre mice. These data support the conclusion that the immune system employs ATLOs to organize aorta T cell homeostasis during aging and that VSMC-LTβRs participate in atherosclerosis protection via ATLOs.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

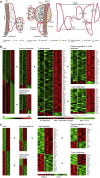
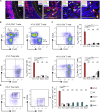
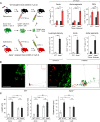
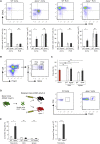
Comment in
-
Another TLO in the Wall: Education and Control of T Cells in Atherosclerotic Arteries.Immunity. 2015 Jun 16;42(6):981-3. doi: 10.1016/j.immuni.2015.05.022. Immunity. 2015. PMID: 26084016
Similar articles
-
Artery Tertiary Lymphoid Organs Control Multilayered Territorialized Atherosclerosis B-Cell Responses in Aged ApoE-/- Mice.Arterioscler Thromb Vasc Biol. 2016 Jun;36(6):1174-85. doi: 10.1161/ATVBAHA.115.306983. Epub 2016 Apr 21. Arterioscler Thromb Vasc Biol. 2016. PMID: 27102965 Free PMC article.
-
Another TLO in the Wall: Education and Control of T Cells in Atherosclerotic Arteries.Immunity. 2015 Jun 16;42(6):981-3. doi: 10.1016/j.immuni.2015.05.022. Immunity. 2015. PMID: 26084016
-
Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE-/- mice.J Exp Med. 2009 Jan 16;206(1):233-48. doi: 10.1084/jem.20080752. Epub 2009 Jan 12. J Exp Med. 2009. PMID: 19139167 Free PMC article.
-
Artery tertiary lymphoid organs contribute to innate and adaptive immune responses in advanced mouse atherosclerosis.Circ Res. 2014 May 23;114(11):1772-87. doi: 10.1161/CIRCRESAHA.114.301137. Circ Res. 2014. PMID: 24855201 Review.
-
Artery Tertiary Lymphoid Organs: Powerhouses of Atherosclerosis Immunity.Front Immunol. 2016 Oct 10;7:387. doi: 10.3389/fimmu.2016.00387. eCollection 2016. Front Immunol. 2016. PMID: 27777573 Free PMC article. Review.
Cited by
-
The Evolving Role of Dendritic Cells in Atherosclerosis.Int J Mol Sci. 2024 Feb 19;25(4):2450. doi: 10.3390/ijms25042450. Int J Mol Sci. 2024. PMID: 38397127 Free PMC article. Review.
-
Crosstalk between dendritic cells and T lymphocytes during atherogenesis: Focus on antigen presentation and break of tolerance.Front Cardiovasc Med. 2022 Jul 28;9:934314. doi: 10.3389/fcvm.2022.934314. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35966516 Free PMC article. Review.
-
The Impact of the Nervous System on Arteries and the Heart: The Neuroimmune Cardiovascular Circuit Hypothesis.Cells. 2023 Oct 19;12(20):2485. doi: 10.3390/cells12202485. Cells. 2023. PMID: 37887328 Free PMC article.
-
De-stressing plaques attenuates atherosclerosis progression.Trends Immunol. 2022 Aug;43(8):601-603. doi: 10.1016/j.it.2022.06.008. Epub 2022 Jul 12. Trends Immunol. 2022. PMID: 35840527 Free PMC article.
-
Type 3 innate lymphoid cell-derived lymphotoxin prevents microbiota-dependent inflammation.Cell Mol Immunol. 2018 Jul;15(7):697-709. doi: 10.1038/cmi.2017.25. Epub 2017 Jun 5. Cell Mol Immunol. 2018. PMID: 28579615 Free PMC article.
References
-
- Ait-Oufella H., Salomon B.L., Potteaux S., Robertson A.K., Gourdy P., Zoll J., Merval R., Esposito B., Cohen J.L., Fisson S. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 2006;12:178–180. - PubMed
-
- Aloisi F., Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 2006;6:205–217. - PubMed
-
- Bilate A.M., Lafaille J.J. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu. Rev. Immunol. 2012;30:733–758. - PubMed
-
- Boucher P., Gotthardt M., Li W.P., Anderson R.G., Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. - PubMed
-
- Cao R.Y., St Amand T., Grabner R., Habenicht A.J., Funk C.D. Genetic and pharmacological inhibition of the 5-lipoxygenase/leukotriene pathway in atherosclerotic lesion development in ApoE deficient mice. Atherosclerosis. 2009;203:395–400. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

