Human miR-3145 inhibits influenza A viruses replication by targeting and silencing viral PB1 gene
- PMID: 26080461
- PMCID: PMC4935342
- DOI: 10.1177/1535370215589051
Human miR-3145 inhibits influenza A viruses replication by targeting and silencing viral PB1 gene
Abstract
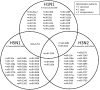
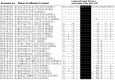
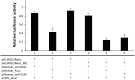
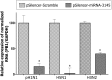
MicroRNAs (miRNAs) play an important role in the regulation of gene expression and are involved in many cellular processes including inhibition of viral replication in infected cells. In this study, three subtypes of influenza A viruses (pH1N1, H5N1 and H3N2) were analyzed to identify candidate human miRNAs targeting and silencing viral genes expression. Candidate human miRNAs were predicted by miRBase and RNAhybrid based on minimum free energy (MFE) and hybridization patterns between human miRNAs and viral target genes. In silico analysis presented 76 miRNAs targeting influenza A viruses, including 70 miRNAs that targeted specific subtypes (21 for pH1N1, 27 for H5N1 and 22 for H3N2) and 6 miRNAs (miR-216b, miR-3145, miR-3682, miR-4513, miR-4753 and miR-5693) that targeted multiple subtypes of influenza A viruses. Interestingly, miR-3145 is the only candidate miRNA targeting all three subtypes of influenza A viruses. The miR-3145 targets to PB1 encoding polymerase basic protein 1, which is the main component of the viral polymerase complex. The silencing effect of miR-3145 was validated by 3'-UTR reporter assay and inhibition of influenza viral replication in A549 cells. In 3'-UTR reporter assay, results revealed that miR-3145 triggered significant reduction of the luciferase activity. Moreover, expression of viral PB1 genes was also inhibited considerably (P value < 0.05) in viral infected cells expressing mimic miR-3145. In conclusion, this study demonstrated that human miR-3145 triggered silencing of viral PB1 genes and lead to inhibition of multiple subtypes of influenza viral replication. Therefore, hsa-miR-3145 might be useful for alternative treatment of influenza A viruses in the future.
Keywords: influenza A virus; inhibition; miR-3145; miRNA; silencing; viral replication.
© 2015 by the Society for Experimental Biology and Medicine.
Figures
Similar articles
-
Human microRNAs profiling in response to influenza A viruses (subtypes pH1N1, H3N2, and H5N1).Exp Biol Med (Maywood). 2016 Feb;241(4):409-20. doi: 10.1177/1535370215611764. Epub 2015 Oct 29. Exp Biol Med (Maywood). 2016. PMID: 26518627 Free PMC article.
-
MicroRNA hsa-miR-324-5p Suppresses H5N1 Virus Replication by Targeting the Viral PB1 and Host CUEDC2.J Virol. 2018 Sep 12;92(19):e01057-18. doi: 10.1128/JVI.01057-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30045983 Free PMC article.
-
The highly pathogenic H5N1 influenza A virus down-regulated several cellular MicroRNAs which target viral genome.J Cell Mol Med. 2017 Nov;21(11):3076-3086. doi: 10.1111/jcmm.13219. Epub 2017 Jun 13. J Cell Mol Med. 2017. PMID: 28609011 Free PMC article.
-
Laninamivir octanoate: a new long-acting neuraminidase inhibitor for the treatment of influenza.Expert Rev Anti Infect Ther. 2011 Oct;9(10):851-7. doi: 10.1586/eri.11.112. Expert Rev Anti Infect Ther. 2011. PMID: 21973296 Review.
-
The epidemiology and spread of drug resistant human influenza viruses.Curr Opin Virol. 2014 Oct;8:22-9. doi: 10.1016/j.coviro.2014.04.009. Epub 2014 May 24. Curr Opin Virol. 2014. PMID: 24866471 Review.
Cited by
-
MiR-130c-5p targets the SHVV n gene and upregulates immune cytokines (IL-6, IL-22, IL-1β) to inhibit viral replication.Front Immunol. 2024 Nov 1;15:1486816. doi: 10.3389/fimmu.2024.1486816. eCollection 2024. Front Immunol. 2024. PMID: 39555085 Free PMC article.
-
MicroRNAs and Long Non-Coding RNAs as Potential Candidates to Target Specific Motifs of SARS-CoV-2.Noncoding RNA. 2021 Feb 18;7(1):14. doi: 10.3390/ncrna7010014. Noncoding RNA. 2021. PMID: 33670580 Free PMC article.
-
Virus-host interaction networks as new antiviral drug targets for IAV and SARS-CoV-2.Emerg Microbes Infect. 2022 Dec;11(1):1371-1389. doi: 10.1080/22221751.2022.2071175. Emerg Microbes Infect. 2022. PMID: 35476817 Free PMC article.
-
Distinct miRNA Profile of Cellular and Extracellular Vesicles Released from Chicken Tracheal Cells Following Avian Influenza Virus Infection.Vaccines (Basel). 2020 Aug 5;8(3):438. doi: 10.3390/vaccines8030438. Vaccines (Basel). 2020. PMID: 32764349 Free PMC article.
-
Exosome therapeutics for COVID-19 and respiratory viruses.View (Beijing). 2021 Jun;2(3):20200186. doi: 10.1002/VIW.20200186. Epub 2021 Jan 31. View (Beijing). 2021. PMID: 34766162 Free PMC article. Review.
References
-
- Centers for Disease C, Prevention. Update: Influenza A (H3N2)v transmission and guidelines – five states, 2011. MMWR Morbid Mortal Weekly Rep 2012; 60: 1741–4. - PubMed
-
- Tran TH, Nguyen TL, Nguyen TD, Luong TS, Pham PM, Nguyen vV, Pham TS, Vo CD, Le TQ, Ngo TT, Dao BK, Le PP, Nguyen TT, Hoang TL, Cao VT, Le TG, Nguyen DT, Le HN, Nguyen KT, Le HS, Le VT, Christiane D, Tran TT, Menno de J, Schultsz C, Cheng P, Lim W, Horby P, Farrar J, World Health Organization International. Avian Influenza Investigative Team Avian influenza A (H5N1) in 10 patients in Vietnam. New Engl J Med 2004; 350: 1179–88. - PubMed
-
- Ambros V. microRNAs: tiny regulators with great potential. Cell 2001; 107: 823–6. - PubMed
-
- Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer 2007; 96 Suppl: R40–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

