Molecular pharmacodynamics of emixustat in protection against retinal degeneration
- PMID: 26075817
- PMCID: PMC4563688
- DOI: 10.1172/JCI80950
Molecular pharmacodynamics of emixustat in protection against retinal degeneration
Abstract
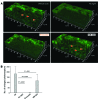
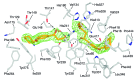
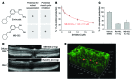
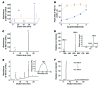
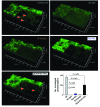
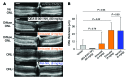
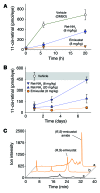
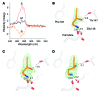
Emixustat is a visual cycle modulator that has entered clinical trials as a treatment for age-related macular degeneration (AMD). This molecule has been proposed to inhibit the visual cycle isomerase RPE65, thereby slowing regeneration of 11-cis-retinal and reducing production of retinaldehyde condensation byproducts that may be involved in AMD pathology. Previously, we reported that all-trans-retinal (atRAL) is directly cytotoxic and that certain primary amine compounds that transiently sequester atRAL via Schiff base formation ameliorate retinal degeneration. Here, we have shown that emixustat stereoselectively inhibits RPE65 by direct active site binding. However, we detected the presence of emixustat-atRAL Schiff base conjugates, indicating that emixustat also acts as a retinal scavenger, which may contribute to its therapeutic effects. Using agents that lack either RPE65 inhibitory activity or the capacity to sequester atRAL, we assessed the relative importance of these 2 modes of action in protection against retinal phototoxicity in mice. The atRAL sequestrant QEA-B-001-NH2 conferred protection against phototoxicity without inhibiting RPE65, whereas an emixustat derivative incapable of atRAL sequestration was minimally protective, despite direct inhibition of RPE65. These data indicate that atRAL sequestration is an essential mechanism underlying the protective effects of emixustat and related compounds against retinal phototoxicity. Moreover, atRAL sequestration should be considered in the design of next-generation visual cycle modulators.
Figures
Similar articles
-
Rational Tuning of Visual Cycle Modulator Pharmacodynamics.J Pharmacol Exp Ther. 2017 Jul;362(1):131-145. doi: 10.1124/jpet.117.240721. Epub 2017 May 5. J Pharmacol Exp Ther. 2017. PMID: 28476927 Free PMC article.
-
Visual Cycle Modulation as an Approach toward Preservation of Retinal Integrity.PLoS One. 2015 May 13;10(5):e0124940. doi: 10.1371/journal.pone.0124940. eCollection 2015. PLoS One. 2015. PMID: 25970164 Free PMC article.
-
All-trans-Retinaldehyde Contributes to Retinal Vascular Permeability in Ischemia Reperfusion.Invest Ophthalmol Vis Sci. 2020 Jun 3;61(6):8. doi: 10.1167/iovs.61.6.8. Invest Ophthalmol Vis Sci. 2020. PMID: 32492112 Free PMC article.
-
Pharmacotherapy of retinal disease with visual cycle modulators.Expert Opin Pharmacother. 2018 Apr;19(5):471-481. doi: 10.1080/14656566.2018.1448060. Epub 2018 Mar 15. Expert Opin Pharmacother. 2018. PMID: 29542350 Review.
-
Vitamin A and Vision.Subcell Biochem. 2016;81:231-259. doi: 10.1007/978-94-024-0945-1_9. Subcell Biochem. 2016. PMID: 27830507 Review.
Cited by
-
Recent Advances in Age-Related Macular Degeneration Therapies.Molecules. 2022 Aug 10;27(16):5089. doi: 10.3390/molecules27165089. Molecules. 2022. PMID: 36014339 Free PMC article. Review.
-
From mouse to human: Accessing the biochemistry of vision in vivo by two-photon excitation.Prog Retin Eye Res. 2023 Mar;93:101170. doi: 10.1016/j.preteyeres.2023.101170. Epub 2023 Feb 12. Prog Retin Eye Res. 2023. PMID: 36787681 Free PMC article. Review.
-
Systemic administration of the di-apocarotenoid norbixin (BIO201) is neuroprotective, preserves photoreceptor function and inhibits A2E and lipofuscin accumulation in animal models of age-related macular degeneration and Stargardt disease.Aging (Albany NY). 2020 Apr 7;12(7):6151-6171. doi: 10.18632/aging.103014. Epub 2020 Apr 7. Aging (Albany NY). 2020. PMID: 32255762 Free PMC article.
-
Impaired Rhodopsin Generation in the Rat Model of Diabetic Retinopathy.Am J Pathol. 2017 Oct;187(10):2222-2231. doi: 10.1016/j.ajpath.2017.06.007. Epub 2017 Jul 19. Am J Pathol. 2017. PMID: 28734946 Free PMC article.
-
A novel small molecule chaperone of rod opsin and its potential therapy for retinal degeneration.Nat Commun. 2018 May 17;9(1):1976. doi: 10.1038/s41467-018-04261-1. Nat Commun. 2018. PMID: 29773803 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- EY023948/EY/NEI NIH HHS/United States
- R01 EY009339/EY/NEI NIH HHS/United States
- P41 GM103403/GM/NIGMS NIH HHS/United States
- EY025451/EY/NEI NIH HHS/United States
- AG043645/AG/NIA NIH HHS/United States
- U01 EY025451/EY/NEI NIH HHS/United States
- CA157735/CA/NCI NIH HHS/United States
- IK2 BX002683/BX/BLRD VA/United States
- R01 EY023948/EY/NEI NIH HHS/United States
- R44 AG043645/AG/NIA NIH HHS/United States
- EY009339/EY/NEI NIH HHS/United States
- EY021126/EY/NEI NIH HHS/United States
- R24 EY021126/EY/NEI NIH HHS/United States
- R01 CA157735/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

