Mesaconase Activity of Class I Fumarase Contributes to Mesaconate Utilization by Burkholderia xenovorans
- PMID: 26070669
- PMCID: PMC4510160
- DOI: 10.1128/AEM.00822-15
Mesaconase Activity of Class I Fumarase Contributes to Mesaconate Utilization by Burkholderia xenovorans
Abstract
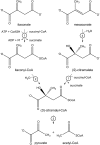
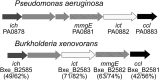
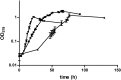
Pseudomonas aeruginosa, Yersinia pestis, and many other bacteria are able to utilize the C5-dicarboxylic acid itaconate (methylenesuccinate). Itaconate degradation starts with its activation to itaconyl coenzyme A (itaconyl-CoA), which is further hydrated to (S)-citramalyl-CoA, and citramalyl-CoA is finally cleaved into acetyl-CoA and pyruvate. The xenobiotic-degrading betaproteobacterium Burkholderia xenovorans possesses a P. aeruginosa-like itaconate degradation gene cluster and is able to grow on itaconate and its isomer mesaconate (methylfumarate). Although itaconate degradation proceeds in B. xenovorans in the same way as in P. aeruginosa, the pathway of mesaconate utilization is not known. Here, we show that mesaconate is metabolized through its hydration to (S)-citramalate. The latter compound is then metabolized to acetyl-CoA and pyruvate with the participation of two enzymes of the itaconate degradation pathway, a promiscuous itaconate-CoA transferase able to activate (S)-citramalate in addition to itaconate and (S)-citramalyl-CoA lyase. The first reaction of the pathway, the mesaconate hydratase (mesaconase) reaction, is catalyzed by a class I fumarase. As this enzyme (Bxe_A3136) has similar efficiencies (kcat/Km) for both fumarate and mesaconate hydration, we conclude that B. xenovorans class I fumarase is in fact a promiscuous fumarase/mesaconase. This promiscuity is physiologically relevant, as it allows the growth of this bacterium on mesaconate as a sole carbon and energy source.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Mesaconase/Fumarase FumD in Escherichia coli O157:H7 and Promiscuity of Escherichia coli Class I Fumarases FumA and FumB.PLoS One. 2015 Dec 14;10(12):e0145098. doi: 10.1371/journal.pone.0145098. eCollection 2015. PLoS One. 2015. PMID: 26658641 Free PMC article.
-
Bacterial itaconate degradation promotes pathogenicity.Nat Chem Biol. 2014 May;10(5):371-7. doi: 10.1038/nchembio.1482. Epub 2014 Mar 23. Nat Chem Biol. 2014. PMID: 24657929
-
Physiological roles of two enzymes with fumarase activity in two Pseudomonads.Biochem Int. 1987 May;14(5):871-8. Biochem Int. 1987. PMID: 3136773
-
Unfamiliar metabolic links in the central carbon metabolism.J Biotechnol. 2014 Dec 20;192 Pt B:314-22. doi: 10.1016/j.jbiotec.2014.02.015. Epub 2014 Feb 24. J Biotechnol. 2014. PMID: 24576434 Review.
-
[Mitochondrial fumarase].Nihon Rinsho. 2002 Apr;60 Suppl 4:126-9. Nihon Rinsho. 2002. PMID: 12013833 Review. Japanese. No abstract available.
Cited by
-
Improvement of dicarboxylic acid production with Methylorubrum extorquens by reduction of product reuptake.Appl Microbiol Biotechnol. 2022 Oct;106(19-20):6713-6731. doi: 10.1007/s00253-022-12161-0. Epub 2022 Sep 15. Appl Microbiol Biotechnol. 2022. PMID: 36104545 Free PMC article.
-
Metabolomics Analysis for Nitrite Degradation by the Metabolites of Limosilactobacillus fermentum RC4.Foods. 2022 Mar 30;11(7):1009. doi: 10.3390/foods11071009. Foods. 2022. PMID: 35407096 Free PMC article.
-
Enantioselectivity in the enzymatic dehydration of malate and tartrate: Mirror image specificities of structurally similar dehydratases.Protein Sci. 2023 Oct;32(10):e4779. doi: 10.1002/pro.4779. Protein Sci. 2023. PMID: 37695939 Free PMC article.
-
Crystal structure of an Fe-S cluster-containing fumarate hydratase enzyme from Leishmania major reveals a unique protein fold.Proc Natl Acad Sci U S A. 2016 Aug 30;113(35):9804-9. doi: 10.1073/pnas.1605031113. Epub 2016 Aug 15. Proc Natl Acad Sci U S A. 2016. PMID: 27528683 Free PMC article.
-
Characterization and engineering of branched short-chain dicarboxylate metabolism in Pseudomonas reveals resistance to fungal 2-hydroxyparaconate.Metab Eng. 2023 Jan;75:205-216. doi: 10.1016/j.ymben.2022.12.008. Epub 2022 Dec 26. Metab Eng. 2023. PMID: 36581064 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

