Ubiquitin-specific Protease 15 Negatively Regulates Virus-induced Type I Interferon Signaling via Catalytically-dependent and -independent Mechanisms
- PMID: 26061460
- PMCID: PMC4650652
- DOI: 10.1038/srep11220
Ubiquitin-specific Protease 15 Negatively Regulates Virus-induced Type I Interferon Signaling via Catalytically-dependent and -independent Mechanisms
Abstract
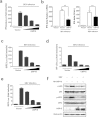
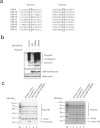
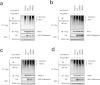
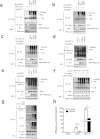
Viral infection triggers a series of signaling cascades, which converge to activate the transcription factors nuclear factor-κB (NF-κB) and interferon regulatory factor 3 (IRF3), thereby inducing the transcription of type I interferons (IFNs). Although not fully characterized, these innate antiviral responses are fine-tuned by dynamic ubiquitination and deubiquitination processes. In this study, we report ubiquitin-specific protease (USP) 15 is involved in regulation of the retinoic acid-inducible gene I (RIG-I)-dependent type I IFN induction pathway. Knockdown of endogenous USP15 augmented cellular antiviral responses. Overexpression of USP15 inhibited the transcription of IFN-β. Further analyses identified histidine 862 as a critical residue for USP15's catalytic activity. Interestingly, USP15 specifically removed lysine 63-linked polyubiquitin chains from RIG-I among the essential components in RIG-I-like receptor-dependent pathway. In addition, we demonstrated that in contrast to USP15 de-ubiquitinating (DUB) activity, USP15-mediated inhibition of IFN signaling was not abolished by mutations eliminating the catalytic activity, indicating that a fraction of USP15-mediated IFN antagonism was independent of the DUB activity. Catalytically inactive USP15 mutants, as did the wild-type protein, disrupted virus-induced interaction of RIG-I and IFN-β promoter stimulator 1. Taken together, our data demonstrate that USP15 acts as a negative regulator of RIG-I signaling via DUB-dependent and independent mechanisms.
Figures


Similar articles
-
The ubiquitin-specific protease USP15 promotes RIG-I-mediated antiviral signaling by deubiquitylating TRIM25.Sci Signal. 2014 Jan 7;7(307):ra3. doi: 10.1126/scisignal.2004577. Sci Signal. 2014. PMID: 24399297 Free PMC article.
-
The Human Papillomavirus E6 Oncoprotein Targets USP15 and TRIM25 To Suppress RIG-I-Mediated Innate Immune Signaling.J Virol. 2018 Feb 26;92(6):e01737-17. doi: 10.1128/JVI.01737-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29263274 Free PMC article.
-
USP3 inhibits type I interferon signaling by deubiquitinating RIG-I-like receptors.Cell Res. 2014 Apr;24(4):400-16. doi: 10.1038/cr.2013.170. Epub 2013 Dec 24. Cell Res. 2014. PMID: 24366338 Free PMC article.
-
The Regulations of Deubiquitinase USP15 and Its Pathophysiological Mechanisms in Diseases.Int J Mol Sci. 2017 Feb 24;18(3):483. doi: 10.3390/ijms18030483. Int J Mol Sci. 2017. PMID: 28245560 Free PMC article. Review.
-
USP15: a review of its implication in immune and inflammatory processes and tumor progression.Genes Immun. 2021 May;22(1):12-23. doi: 10.1038/s41435-021-00125-9. Epub 2021 Apr 6. Genes Immun. 2021. PMID: 33824497 Review.
Cited by
-
The Role of Deubiquitinases in Virus Replication and Host Innate Immune Response.Front Microbiol. 2022 Feb 24;13:839624. doi: 10.3389/fmicb.2022.839624. eCollection 2022. Front Microbiol. 2022. PMID: 35283827 Free PMC article. Review.
-
USP15 regulates dynamic protein-protein interactions of the spliceosome through deubiquitination of PRP31.Nucleic Acids Res. 2017 May 5;45(8):4866-4880. doi: 10.1093/nar/gkw1365. Nucleic Acids Res. 2017. PMID: 28088760 Free PMC article.
-
Negative regulators of the RIG-I-like receptor signaling pathway.Eur J Immunol. 2017 Apr;47(4):615-628. doi: 10.1002/eji.201646484. Eur J Immunol. 2017. PMID: 28295214 Free PMC article. Review.
-
The Roles of TRIMs in Antiviral Innate Immune Signaling.Front Cell Infect Microbiol. 2021 Mar 15;11:628275. doi: 10.3389/fcimb.2021.628275. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33791238 Free PMC article. Review.
-
Pristimerin suppresses AIM2 inflammasome by modulating AIM2-PYCARD/ASC stability via selective autophagy to alleviate tendinopathy.Autophagy. 2024 Jan;20(1):76-93. doi: 10.1080/15548627.2023.2249392. Epub 2023 Aug 30. Autophagy. 2024. PMID: 37647255 Free PMC article.
References
-
- Akira S., Uematsu S. & Takeuchi O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006). - PubMed
-
- Saito T. & Gale M. Jr. Principles of intracellular viral recognition. Curr. Opin. Immunol. 19, 17–23 (2007). - PubMed
-
- Takeuchi O. & Akira S. MDA5/RIG-I and virus recognition. Curr. Opin. Immunol. 20, 17–22 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

