Contribution of the Major ND10 Proteins PML, hDaxx and Sp100 to the Regulation of Human Cytomegalovirus Latency and Lytic Replication in the Monocytic Cell Line THP-1
- PMID: 26057166
- PMCID: PMC4488718
- DOI: 10.3390/v7062751
Contribution of the Major ND10 Proteins PML, hDaxx and Sp100 to the Regulation of Human Cytomegalovirus Latency and Lytic Replication in the Monocytic Cell Line THP-1
Abstract
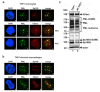
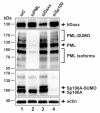
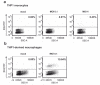
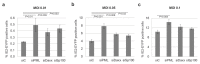
Promyelocytic leukemia nuclear bodies, also termed nuclear domain 10 (ND10), have emerged as nuclear protein accumulations mediating an intrinsic cellular defense against viral infections via chromatin-based mechanisms, however, their contribution to the control of herpesviral latency is still controversial. In this study, we utilized the monocytic cell line THP-1 as an in vitro latency model for human cytomegalovirus infection (HCMV). Characterization of THP-1 cells by immunofluorescence andWestern blot analysis confirmed the expression of all major ND10 components. THP-1 cells with a stable, individual knockdown of PML, hDaxx or Sp100 were generated. Importantly, depletion of the major ND10 proteins did not prevent the terminal cellular differentiation of THP-1 monocytes. After construction of a recombinant, endotheliotropic human cytomegalovirus expressing IE2-EYFP, we investigated whether the depletion of ND10 proteins affects the onset of viral IE gene expression. While after infection of differentiated, THP-1-derived macrophages as well as during differentiation-induced reactivation from latency an increase in the number of IE-expressing cells was readily detectable in the absence of the major ND10 proteins, no effect was observed in non-differentiated monocytes. We conclude that PML, hDaxx and Sp100 primarily act as cellular restriction factors during lytic HCMV replication and during the dynamic process of reactivation but do not serve as key determinants for the establishment of HCMV latency.
Keywords: ND10; PML; cytomegalovirus; intrinsic immunity; latency; restriction factor.
Figures




Similar articles
-
Evidence for a role of the cellular ND10 protein PML in mediating intrinsic immunity against human cytomegalovirus infections.J Virol. 2006 Aug;80(16):8006-18. doi: 10.1128/JVI.00743-06. J Virol. 2006. PMID: 16873257 Free PMC article.
-
Components of promyelocytic leukemia nuclear bodies (ND10) act cooperatively to repress herpesvirus infection.J Virol. 2013 Feb;87(4):2174-85. doi: 10.1128/JVI.02950-12. Epub 2012 Dec 5. J Virol. 2013. PMID: 23221561 Free PMC article.
-
Viral FGARAT Homolog ORF75 of Rhesus Monkey Rhadinovirus Effects Proteasomal Degradation of the ND10 Components SP100 and PML.J Virol. 2016 Aug 12;90(17):8013-28. doi: 10.1128/JVI.01181-16. Print 2016 Sep 1. J Virol. 2016. PMID: 27356898 Free PMC article.
-
Intrinsic cellular defense mechanisms targeting human cytomegalovirus.Virus Res. 2011 May;157(2):128-33. doi: 10.1016/j.virusres.2010.10.002. Epub 2010 Oct 8. Virus Res. 2011. PMID: 20934469 Review.
-
Emerging Role of PML Nuclear Bodies in Innate Immune Signaling.J Virol. 2016 Jun 10;90(13):5850-5854. doi: 10.1128/JVI.01979-15. Print 2016 Jul 1. J Virol. 2016. PMID: 27053550 Free PMC article. Review.
Cited by
-
The chromatin remodeling protein ATRX positively regulates IRF3-dependent type I interferon production and interferon-induced gene expression.PLoS Pathog. 2022 Aug 8;18(8):e1010748. doi: 10.1371/journal.ppat.1010748. eCollection 2022 Aug. PLoS Pathog. 2022. PMID: 35939517 Free PMC article.
-
Intracellular Antiviral Immunity.Adv Virus Res. 2018;100:309-354. doi: 10.1016/bs.aivir.2018.01.002. Epub 2018 Feb 16. Adv Virus Res. 2018. PMID: 29551141 Free PMC article. Review.
-
Combinatorial Drug Treatments Reveal Promising Anticytomegaloviral Profiles for Clinically Relevant Pharmaceutical Kinase Inhibitors (PKIs).Int J Mol Sci. 2021 Jan 8;22(2):575. doi: 10.3390/ijms22020575. Int J Mol Sci. 2021. PMID: 33430060 Free PMC article.
-
Epigenetic Restriction Factors (eRFs) in Virus Infection.Viruses. 2024 Jan 25;16(2):183. doi: 10.3390/v16020183. Viruses. 2024. PMID: 38399958 Free PMC article. Review.
-
The Golgi sorting motifs of human cytomegalovirus UL138 are not required for latency maintenance.Virus Res. 2019 Sep;270:197646. doi: 10.1016/j.virusres.2019.197646. Epub 2019 Jun 28. Virus Res. 2019. PMID: 31260705 Free PMC article.
References
-
- Bolovan-Fritts C.A., Mocarski E.S., Wiedeman J.A. Peripheral blood CD14(+) cells from healthy subjects carry a circular conformation of latent cytomegalovirus genome. Blood. 1999;93:394–398. - PubMed
-
- Khaiboullina S.F., Maciejewski J.P., Crapnell K., Spallone P.A., Dean S.A., Pari G.S., Zanjani E.D., Jeor S.S. Human cytomegalovirus persists in myeloid progenitors and is passed to the myeloid progeny in a latent form. Br. J. Haematol. 2004;126:410–417. doi: 10.1111/j.1365-2141.2004.05056.x. - DOI - PubMed
-
- Sindre H., Tjoonnfjord G.E., Rollag H., Ranneberg-Nilsen T., Veiby O.P., Beck S., Degre M., Hestdal K. Human cytomegalovirus suppression of and latency in early hematopoietic progenitor cells. Blood. 1996;88:4526–4533. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

