mTORC1 Down-Regulates Cyclin-Dependent Kinase 8 (CDK8) and Cyclin C (CycC)
- PMID: 26042770
- PMCID: PMC4456374
- DOI: 10.1371/journal.pone.0126240
mTORC1 Down-Regulates Cyclin-Dependent Kinase 8 (CDK8) and Cyclin C (CycC)
Abstract
In non-alcoholic fatty liver disease (NAFLD) and insulin resistance, hepatic de novo lipogenesis is often elevated, but the underlying mechanisms remain poorly understood. Recently, we show that CDK8 functions to suppress de novo lipogenesis. Here, we identify the mammalian target of rapamycin complex 1 (mTORC1) as a critical regulator of CDK8 and its activating partner CycC. Using pharmacologic and genetic approaches, we show that increased mTORC1 activation causes the reduction of the CDK8-CycC complex in vitro and in mouse liver in vivo. In addition, mTORC1 is more active in three mouse models of NAFLD, correlated with the lower abundance of the CDK8-CycC complex. Consistent with the inhibitory role of CDK8 on de novo lipogenesis, nuclear SREBP-1c proteins and lipogenic enzymes are accumulated in NAFLD models. Thus, our results suggest that mTORC1 activation in NAFLD and insulin resistance results in down-regulation of the CDK8-CycC complex and elevation of lipogenic protein expression.
Conflict of interest statement
Figures


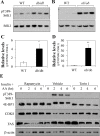
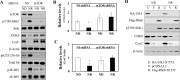

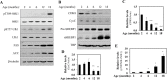
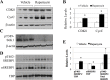
Similar articles
-
Regulation of lipogenesis by cyclin-dependent kinase 8-mediated control of SREBP-1.J Clin Invest. 2012 Jul;122(7):2417-27. doi: 10.1172/JCI61462. Epub 2012 Jun 11. J Clin Invest. 2012. PMID: 22684109 Free PMC article.
-
CDK8-Cyclin C Mediates Nutritional Regulation of Developmental Transitions through the Ecdysone Receptor in Drosophila.PLoS Biol. 2015 Jul 29;13(7):e1002207. doi: 10.1371/journal.pbio.1002207. eCollection 2015 Jul. PLoS Biol. 2015. PMID: 26222308 Free PMC article.
-
Livers with constitutive mTORC1 activity resist steatosis independent of feedback suppression of Akt.PLoS One. 2015 Feb 3;10(2):e0117000. doi: 10.1371/journal.pone.0117000. eCollection 2015. PLoS One. 2015. PMID: 25646773 Free PMC article.
-
Connecting mTORC1 signaling to SREBP-1 activation.Curr Opin Lipidol. 2012 Jun;23(3):226-234. doi: 10.1097/MOL.0b013e328352dd03. Curr Opin Lipidol. 2012. PMID: 22449814 Review.
-
Dysregulation of CDK8 and Cyclin C in tumorigenesis.J Genet Genomics. 2011 Oct 20;38(10):439-52. doi: 10.1016/j.jgg.2011.09.002. Epub 2011 Sep 16. J Genet Genomics. 2011. PMID: 22035865 Free PMC article. Review.
Cited by
-
Lipogenic SREBP-1a/c transcription factors activate expression of the iron regulator hepcidin, revealing cross-talk between lipid and iron metabolisms.J Biol Chem. 2019 Aug 23;294(34):12743-12753. doi: 10.1074/jbc.RA119.009644. Epub 2019 Jul 3. J Biol Chem. 2019. PMID: 31270208 Free PMC article.
-
Cyclin C regulates adipogenesis by stimulating transcriptional activity of CCAAT/enhancer-binding protein α.J Biol Chem. 2017 May 26;292(21):8918-8932. doi: 10.1074/jbc.M117.776229. Epub 2017 Mar 28. J Biol Chem. 2017. PMID: 28351837 Free PMC article.
-
Regulation of metabolism by the Mediator complex.Biophys Rep. 2016;2(2):69-77. doi: 10.1007/s41048-016-0031-6. Epub 2016 Nov 1. Biophys Rep. 2016. PMID: 28018965 Free PMC article.
-
Nonalcoholic Fatty Liver Disease and Staging of Hepatic Fibrosis.Adv Exp Med Biol. 2024;1460:539-574. doi: 10.1007/978-3-031-63657-8_18. Adv Exp Med Biol. 2024. PMID: 39287864 Review.
-
Unveiling the impact of CDK8 on tumor progression: mechanisms and therapeutic strategies.Front Pharmacol. 2024 Mar 28;15:1386929. doi: 10.3389/fphar.2024.1386929. eCollection 2024. Front Pharmacol. 2024. PMID: 38606172 Free PMC article. Review.
References
-
- Ferre P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res. 2007;68(2):72–82. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

