ER-endosome contact sites: molecular compositions and functions
- PMID: 26041457
- PMCID: PMC4547891
- DOI: 10.15252/embj.201591481
ER-endosome contact sites: molecular compositions and functions
Abstract
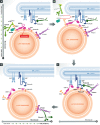
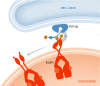
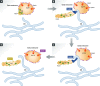
Recent studies have revealed the existence of numerous contact sites between the endoplasmic reticulum (ER) and endosomes in mammalian cells. Such contacts increase during endosome maturation and play key roles in cholesterol transfer, endosome positioning, receptor dephosphorylation, and endosome fission. At least 7 distinct contact sites between the ER and endosomes have been identified to date, which have diverse molecular compositions. Common to these contact sites is that they impose a close apposition between the ER and endosome membranes, which excludes membrane fusion while allowing the flow of molecular signals between the two membranes, in the form of enzymatic modifications, or ion, lipid, or protein transfer. Thus, ER-endosome contact sites ensure coordination of molecular activities between the two compartments while keeping their general compositions intact. Here, we review the molecular architectures and cellular functions of known ER-endosome contact sites and discuss their implications for human health.
Keywords: endoplasmic reticulum; endosome; membrane contact sites.
© 2015 The Authors.
Figures



Similar articles
-
ER-endosome contact sites in endosome positioning and protrusion outgrowth.Biochem Soc Trans. 2016 Apr 15;44(2):441-6. doi: 10.1042/BST20150246. Biochem Soc Trans. 2016. PMID: 27068952 Review.
-
Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth.Nature. 2015 Apr 9;520(7546):234-8. doi: 10.1038/nature14359. Nature. 2015. PMID: 25855459
-
Small regulators, major consequences - Ca²⁺ and cholesterol at the endosome-ER interface.J Cell Sci. 2014 Mar 1;127(Pt 5):929-38. doi: 10.1242/jcs.137539. Epub 2014 Feb 19. J Cell Sci. 2014. PMID: 24554437 Review.
-
Touché! STARD3 and STARD3NL tether the ER to endosomes.Biochem Soc Trans. 2016 Apr 15;44(2):493-8. doi: 10.1042/BST20150269. Biochem Soc Trans. 2016. PMID: 27068960 Review.
-
Faraway, so close! Functions of Endoplasmic reticulum-Endosome contacts.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Jan;1865(1):158490. doi: 10.1016/j.bbalip.2019.06.016. Epub 2019 Jun 26. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 31252175 Review.
Cited by
-
ER as master regulator of membrane trafficking and organelle function.J Cell Biol. 2022 Oct 3;221(10):e202205135. doi: 10.1083/jcb.202205135. Epub 2022 Sep 15. J Cell Biol. 2022. PMID: 36108241 Free PMC article. Review.
-
Fatty acids abrogate the growth-suppressive effects induced by inhibition of cholesterol flux in pancreatic cancer cells.Cancer Cell Int. 2023 Nov 17;23(1):276. doi: 10.1186/s12935-023-03138-8. Cancer Cell Int. 2023. PMID: 37978383 Free PMC article.
-
Annexins-Coordinators of Cholesterol Homeostasis in Endocytic Pathways.Int J Mol Sci. 2018 May 12;19(5):1444. doi: 10.3390/ijms19051444. Int J Mol Sci. 2018. PMID: 29757220 Free PMC article. Review.
-
Staying in touch with the endocytic network: The importance of contacts for cholesterol transport.Traffic. 2020 May;21(5):354-363. doi: 10.1111/tra.12726. Epub 2020 Mar 31. Traffic. 2020. PMID: 32129938 Free PMC article. Review.
-
Adenovirus Modulates Toll-Like Receptor 4 Signaling by Reprogramming ORP1L-VAP Protein Contacts for Cholesterol Transport from Endosomes to the Endoplasmic Reticulum.J Virol. 2017 Feb 28;91(6):e01904-16. doi: 10.1128/JVI.01904-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28077646 Free PMC article.
References
-
- Alpy F, Latchumanan VK, Kedinger V, Janoshazi A, Thiele C, Wendling C, Rio MC, Tomasetto C. Functional characterization of the MENTAL domain. J Biol Chem. 2005;280:17945–17952. - PubMed
-
- Alpy F, Tomasetto C. MLN64 and MENTHO, two mediators of endosomal cholesterol transport. Biochem Soc Trans. 2006;34:343–345. - PubMed
-
- Alpy F, Rousseau A, Schwab Y, Legueux F, Stoll I, Wendling C, Spiegelhalter C, Kessler P, Mathelin C, Rio MC, Levine TP, Tomasetto C. STARD3 or STARD3NL and VAP form a novel molecular tether between late endosomes and the ER. J Cell Sci. 2013;126:5500–5512. - PubMed
-
- Chu BB, Liao YC, Qi W, Xie C, Du X, Wang J, Yang H, Miao HH, Li BL, Song BL. Cholesterol Transport through Lysosome-Peroxisome Membrane Contacts. Cell. 2015;161:291–306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

