ADF/cofilin: a crucial regulator of synapse physiology and behavior
- PMID: 26037722
- PMCID: PMC11113150
- DOI: 10.1007/s00018-015-1941-z
ADF/cofilin: a crucial regulator of synapse physiology and behavior
Abstract
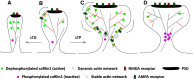
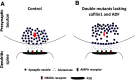
Actin filaments (F-actin) are the major structural component of excitatory synapses, being present in presynaptic terminals and in postsynaptic dendritic spines. In the last decade, it has been appreciated that actin dynamics, the assembly and disassembly of F-actin, is crucial not only for the structure of excitatory synapses, but also for pre- and postsynaptic physiology. Hence, regulators of actin dynamics take a central role in mediating neurotransmitter release, synaptic plasticity, and ultimately behavior. Actin depolymerizing proteins of the ADF/cofilin family are essential regulators of actin dynamics, and a number of recent studies highlighted their crucial functions in excitatory synapses. In dendritic spines, ADF/cofilin activity is required for spine enlargement during initial long-term potentiation (LTP), but needs to be switched off during spine stabilization and LTP consolidation. Conversely, active ADF/cofilin is needed for spine pruning during long-term depression (LTD). Moreover, ADF/cofilin controls activity-induced synaptic availability of glutamate receptors, and exocytosis of synaptic vesicles. These data show that the activity of ADF/cofilin in synapses needs to be spatially and temporally tightly controlled through several upstream regulatory pathways, which have been identified recently. Hence, ADF/cofilin-controlled actin dynamics emerged as a critical and central regulator of synapse physiology. In this review, I will summarize and discuss our current knowledge on the roles of ADF/cofilin in synapse physiology and behavior, by focusing on excitatory synapses of the mammalian central nervous system.
Figures
Similar articles
-
Novel functions for ADF/cofilin in excitatory synapses - lessons from gene-targeted mice.Commun Integr Biol. 2015 Dec 4;8(6):e1114194. doi: 10.1080/19420889.2015.1114194. eCollection 2015 Nov-Dec. Commun Integr Biol. 2015. PMID: 27066177 Free PMC article.
-
ADF/Cofilin Controls Synaptic Actin Dynamics and Regulates Synaptic Vesicle Mobilization and Exocytosis.Cereb Cortex. 2015 Sep;25(9):2863-75. doi: 10.1093/cercor/bhu081. Epub 2014 Apr 25. Cereb Cortex. 2015. PMID: 24770705
-
ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity.Nat Neurosci. 2010 Oct;13(10):1208-15. doi: 10.1038/nn.2634. Epub 2010 Sep 12. Nat Neurosci. 2010. PMID: 20835250 Free PMC article.
-
Regulation of spine morphology and synaptic function by LIMK and the actin cytoskeleton.Rev Neurosci. 2003;14(3):233-40. doi: 10.1515/revneuro.2003.14.3.233. Rev Neurosci. 2003. PMID: 14513866 Review.
-
The Role of ADF/Cofilin in Synaptic Physiology and Alzheimer's Disease.Front Cell Dev Biol. 2020 Nov 12;8:594998. doi: 10.3389/fcell.2020.594998. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33282872 Free PMC article. Review.
Cited by
-
Synaptotoxicity in Alzheimer's Disease Involved a Dysregulation of Actin Cytoskeleton Dynamics through Cofilin 1 Phosphorylation.J Neurosci. 2018 Nov 28;38(48):10349-10361. doi: 10.1523/JNEUROSCI.1409-18.2018. Epub 2018 Oct 19. J Neurosci. 2018. PMID: 30341179 Free PMC article.
-
Remodeling of the postsynaptic proteome in male mice and marmosets during synapse development.Nat Commun. 2024 Mar 28;15(1):2496. doi: 10.1038/s41467-024-46529-9. Nat Commun. 2024. PMID: 38548776 Free PMC article.
-
Cofilin1 oxidation links oxidative distress to mitochondrial demise and neuronal cell death.Cell Death Dis. 2021 Oct 16;12(11):953. doi: 10.1038/s41419-021-04242-1. Cell Death Dis. 2021. PMID: 34657120 Free PMC article.
-
Neuroligin 2 governs synaptic morphology and function through RACK1-cofilin signaling in Drosophila.Commun Biol. 2023 Oct 18;6(1):1056. doi: 10.1038/s42003-023-05428-3. Commun Biol. 2023. PMID: 37853189 Free PMC article.
-
The Role of Ephs and Ephrins in Memory Formation.Int J Neuropsychopharmacol. 2016 Apr 20;19(4):pyv106. doi: 10.1093/ijnp/pyv106. Print 2016 Apr. Int J Neuropsychopharmacol. 2016. PMID: 26371183 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

