Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis
- PMID: 26024392
- PMCID: PMC4815980
- DOI: 10.1038/cdd.2015.70
Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis
Abstract
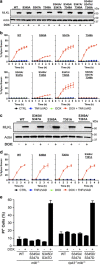
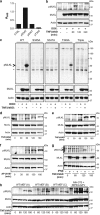
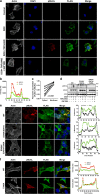
Mixed lineage kinase domain-like pseudokinase (MLKL) mediates necroptosis by translocating to the plasma membrane and inducing its rupture. The activation of MLKL occurs in a multimolecular complex (the 'necrosome'), which is comprised of MLKL, receptor-interacting serine/threonine kinase (RIPK)-3 (RIPK3) and, in some cases, RIPK1. Within this complex, RIPK3 phosphorylates the activation loop of MLKL, promoting conformational changes and allowing the formation of MLKL oligomers, which migrate to the plasma membrane. Previous studies suggested that RIPK3 could phosphorylate the murine MLKL activation loop at Ser345, Ser347 and Thr349. Moreover, substitution of the Ser345 for an aspartic acid creates a constitutively active MLKL, independent of RIPK3 function. Here we examine the role of each of these residues and found that the phosphorylation of Ser345 is critical for RIPK3-mediated necroptosis, Ser347 has a minor accessory role and Thr349 seems to be irrelevant. We generated a specific monoclonal antibody to detect phospho-Ser345 in murine cells. Using this antibody, a series of MLKL mutants and a novel RIPK3 inhibitor, we demonstrate that the phosphorylation of Ser345 is not required for the interaction between RIPK3 and MLKL in the necrosome, but is essential for MLKL translocation, accumulation in the plasma membrane, and consequent necroptosis.
Figures


Similar articles
-
Necroptosis signalling is tuned by phosphorylation of MLKL residues outside the pseudokinase domain activation loop.Biochem J. 2015 Oct 15;471(2):255-65. doi: 10.1042/BJ20150678. Epub 2015 Aug 17. Biochem J. 2015. PMID: 26283547
-
A cytosolic heat shock protein 90 and co-chaperone p23 complex activates RIPK3/MLKL during necroptosis of endothelial cells in acute respiratory distress syndrome.J Mol Med (Berl). 2020 Apr;98(4):569-583. doi: 10.1007/s00109-020-01886-y. Epub 2020 Feb 19. J Mol Med (Berl). 2020. PMID: 32072232
-
Casein kinase-1γ1 and 3 stimulate tumor necrosis factor-induced necroptosis through RIPK3.Cell Death Dis. 2019 Dec 4;10(12):923. doi: 10.1038/s41419-019-2146-4. Cell Death Dis. 2019. PMID: 31801942 Free PMC article.
-
The Inflammatory Signal Adaptor RIPK3: Functions Beyond Necroptosis.Int Rev Cell Mol Biol. 2017;328:253-275. doi: 10.1016/bs.ircmb.2016.08.007. Epub 2016 Sep 22. Int Rev Cell Mol Biol. 2017. PMID: 28069136 Free PMC article. Review.
-
Viral-induced neuronal necroptosis: Detrimental to brain function and regulation by necroptosis inhibitors.Biochem Pharmacol. 2023 Jul;213:115591. doi: 10.1016/j.bcp.2023.115591. Epub 2023 May 16. Biochem Pharmacol. 2023. PMID: 37196683 Review.
Cited by
-
Pathological characteristics of axons and alterations of proteomic and lipidomic profiles in midbrain dopaminergic neurodegeneration induced by WDR45-deficiency.Mol Neurodegener. 2024 Aug 26;19(1):62. doi: 10.1186/s13024-024-00746-4. Mol Neurodegener. 2024. PMID: 39183331 Free PMC article.
-
Neonatal obstructive nephropathy induces necroptosis and necroinflammation.Sci Rep. 2019 Dec 9;9(1):18600. doi: 10.1038/s41598-019-55079-w. Sci Rep. 2019. PMID: 31819111 Free PMC article.
-
Identification of MLKL membrane translocation as a checkpoint in necroptotic cell death using Monobodies.Proc Natl Acad Sci U S A. 2020 Apr 14;117(15):8468-8475. doi: 10.1073/pnas.1919960117. Epub 2020 Mar 31. Proc Natl Acad Sci U S A. 2020. PMID: 32234780 Free PMC article.
-
Plasma membrane changes during programmed cell deaths.Cell Res. 2018 Jan;28(1):9-21. doi: 10.1038/cr.2017.133. Epub 2017 Oct 27. Cell Res. 2018. PMID: 29076500 Free PMC article. Review.
-
Regulated Necrotic Cell Death in Alternative Tumor Therapeutic Strategies.Cells. 2020 Dec 17;9(12):2709. doi: 10.3390/cells9122709. Cells. 2020. PMID: 33348858 Free PMC article. Review.
References
-
- Sun L, Wang H, Wang Z, He S, Chen S, Liao D et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012; 148: 213–227. - PubMed
-
- Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 2013; 39: 443–453. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

