Bridging the gap: need for a data repository to support vaccine prioritization efforts
- PMID: 26022565
- PMCID: PMC4465774
- DOI: 10.1016/j.vaccine.2015.02.032
Bridging the gap: need for a data repository to support vaccine prioritization efforts
Abstract
As the mechanisms for discovery, development, and delivery of new vaccines become increasingly complex, strategic planning and priority setting have become ever more crucial. Traditional single value metrics such as disease burden or cost-effectiveness no longer suffice to rank vaccine candidates for development. The Institute of Medicine-in collaboration with the National Academy of Engineering-has developed a novel software system to support vaccine prioritization efforts. The Strategic Multi-Attribute Ranking Tool for Vaccines-SMART Vaccines-allows decision makers to specify their own value structure, selecting from among 28 pre-defined and up to 7 user-defined attributes relevant to the ranking of vaccine candidates. Widespread use of SMART Vaccines will require compilation of a comprehensive data repository for numerous relevant populations-including their demographics, disease burdens and associated treatment costs, as well as characterizing performance features of potential or existing vaccines that might be created, improved, or deployed. While the software contains preloaded data for a modest number of populations, a large gap exists between the existing data and a comprehensive data repository necessary to make full use of SMART Vaccines. While some of these data exist in disparate sources and forms, constructing a data repository will require much new coordination and focus. Finding strategies to bridge the gap to a comprehensive data repository remains the most important task in bringing SMART Vaccines to full fruition, and to support strategic vaccine prioritization efforts in general.
Keywords: Decision making; Disease burden; Population data; Priority setting; Software tool; Vaccine development.
Copyright © 2015 Elsevier Ltd. All rights reserved.
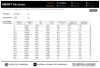
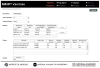
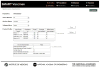
Figures
Similar articles
-
Planning and priority setting for vaccine development and immunization.Vaccine. 2017 Jan 20;35 Suppl 1:A50-A56. doi: 10.1016/j.vaccine.2016.09.072. Epub 2016 Dec 22. Vaccine. 2017. PMID: 28017444 Free PMC article.
-
Strategic Planning in Population Health and Public Health Practice: A Call to Action for Higher Education.Milbank Q. 2016 Mar;94(1):109-25. doi: 10.1111/1468-0009.12182. Milbank Q. 2016. PMID: 26994711 Free PMC article.
-
Informing vaccine decision-making: A strategic multi-attribute ranking tool for vaccines-SMART Vaccines 2.0.Vaccine. 2017 Jan 20;35 Suppl 1:A43-A45. doi: 10.1016/j.vaccine.2016.10.086. Epub 2016 Dec 22. Vaccine. 2017. PMID: 28017435
-
Ranking Vaccines: A Prioritization Framework: Phase I: Demonstration of Concept and a Software Blueprint.Washington (DC): National Academies Press (US); 2012 May 10. Washington (DC): National Academies Press (US); 2012 May 10. PMID: 24851289 Free Books & Documents. Review.
-
Ranking Vaccines: A Prioritization Software Tool: Phase II: Prototype of a Decision-Support System.Washington (DC): National Academies Press (US); 2013 Oct 17. Washington (DC): National Academies Press (US); 2013 Oct 17. PMID: 24901195 Free Books & Documents. Review.
Cited by
-
Planning and priority setting for vaccine development and immunization.Vaccine. 2017 Jan 20;35 Suppl 1:A50-A56. doi: 10.1016/j.vaccine.2016.09.072. Epub 2016 Dec 22. Vaccine. 2017. PMID: 28017444 Free PMC article.
-
Data platforms for open life sciences-A systematic analysis of management instruments.PLoS One. 2022 Oct 25;17(10):e0276204. doi: 10.1371/journal.pone.0276204. eCollection 2022. PLoS One. 2022. PMID: 36282849 Free PMC article.
-
National decision-making for the introduction of new vaccines: A systematic review, 2010-2020.Vaccine. 2021 Apr 1;39(14):1897-1909. doi: 10.1016/j.vaccine.2021.02.059. Epub 2021 Mar 6. Vaccine. 2021. PMID: 33750592 Free PMC article. Review.
-
Strategic Planning in Population Health and Public Health Practice: A Call to Action for Higher Education.Milbank Q. 2016 Mar;94(1):109-25. doi: 10.1111/1468-0009.12182. Milbank Q. 2016. PMID: 26994711 Free PMC article.
-
'It takes two to tango': Bridging the gap between country need and vaccine product innovation.PLoS One. 2020 Jun 10;15(6):e0233950. doi: 10.1371/journal.pone.0233950. eCollection 2020. PLoS One. 2020. PMID: 32520934 Free PMC article.
References
-
- Rappuoli R, Aderem A. A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature. 2011;473(7348):463–9. - PubMed
-
- World Health Organization (WHO) Global Tuberculosis Report 2013. Geneva, Switzerland: 2013.
-
- Colditz GA, Berkey CS, Mosteller F, et al. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995;96(1 Pt 1):29–35. - PubMed
-
- Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367(9517):1173–80. - PubMed
-
- WHO. 23-valent pneumococcal polysaccharide vaccine: WHO Position Paper. Weekly Epidemiological Record. 2008;42:373–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

