Characterization of cooperative bicarbonate uptake into chloroplast stroma in the green alga Chlamydomonas reinhardtii
- PMID: 26015566
- PMCID: PMC4466737
- DOI: 10.1073/pnas.1501659112
Characterization of cooperative bicarbonate uptake into chloroplast stroma in the green alga Chlamydomonas reinhardtii
Abstract
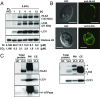
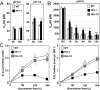
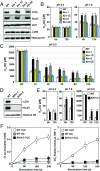
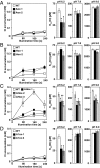
The supply of inorganic carbon (Ci; CO2 and HCO3 (-)) is an environmental rate-limiting factor in aquatic photosynthetic organisms. To overcome the difficulty in acquiring Ci in limiting-CO2 conditions, an active Ci uptake system called the CO2-concentrating mechanism (CCM) is induced to increase CO2 concentrations in the chloroplast stroma. An ATP-binding cassette transporter, HLA3, and a formate/nitrite transporter homolog, LCIA, are reported to be associated with HCO3 (-) uptake [Wang and Spalding (2014) Plant Physiol 166(4):2040-2050]. However, direct evidence of the route of HCO3 (-) uptake from the outside of cells to the chloroplast stroma remains elusive owing to a lack of information on HLA3 localization and comparative analyses of the contribution of HLA3 and LCIA to the CCM. In this study, we revealed that HLA3 and LCIA are localized to the plasma membrane and chloroplast envelope, respectively. Insertion mutants of HLA3 and/or LCIA showed decreased Ci affinities/accumulation, especially in alkaline conditions where HCO3 (-) is the predominant form of Ci. HLA3 and LCIA formed protein complexes independently, and the absence of LCIA decreased HLA3 mRNA accumulation, suggesting the presence of unidentified retrograde signals from the chloroplast to the nucleus to maintain HLA3 mRNA expression. Furthermore, although single overexpression of HLA3 or LCIA in high CO2 conditions did not affect Ci affinity, simultaneous overexpression of HLA3 with LCIA significantly increased Ci affinity/accumulation. These results highlight the HLA3/LCIA-driven cooperative uptake of HCO3 (-) and a key role of LCIA in the maintenance of HLA3 stability as well as Ci affinity/accumulation in the CCM.
Keywords: CO2-concentrating mechanism; Chlamydomonas; bicarbonate uptake; chloroplast envelope; photosynthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Knockdown of limiting-CO2-induced gene HLA3 decreases HCO3- transport and photosynthetic Ci affinity in Chlamydomonas reinhardtii.Proc Natl Acad Sci U S A. 2009 Apr 7;106(14):5990-5. doi: 10.1073/pnas.0812885106. Epub 2009 Mar 24. Proc Natl Acad Sci U S A. 2009. PMID: 19321421 Free PMC article.
-
Chloroplast-mediated regulation of CO2-concentrating mechanism by Ca2+-binding protein CAS in the green alga Chlamydomonas reinhardtii.Proc Natl Acad Sci U S A. 2016 Nov 1;113(44):12586-12591. doi: 10.1073/pnas.1606519113. Epub 2016 Oct 17. Proc Natl Acad Sci U S A. 2016. PMID: 27791081 Free PMC article.
-
Expression activation and functional analysis of HLA3, a putative inorganic carbon transporter in Chlamydomonas reinhardtii.Plant J. 2015 Apr;82(1):1-11. doi: 10.1111/tpj.12788. Plant J. 2015. PMID: 25660294
-
Carbon-concentrating mechanism in a green alga, Chlamydomonas reinhardtii, revealed by transcriptome analyses.J Basic Microbiol. 2009 Feb;49(1):42-51. doi: 10.1002/jobm.200800352. J Basic Microbiol. 2009. PMID: 19253331 Review.
-
Carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii: inorganic carbon transport and CO2 recapture.Photosynth Res. 2011 Sep;109(1-3):115-22. doi: 10.1007/s11120-011-9643-3. Epub 2011 Mar 16. Photosynth Res. 2011. PMID: 21409558 Review.
Cited by
-
Bioengineering of Microalgae: Recent Advances, Perspectives, and Regulatory Challenges for Industrial Application.Front Bioeng Biotechnol. 2020 Sep 3;8:914. doi: 10.3389/fbioe.2020.00914. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33014997 Free PMC article. Review.
-
The pyrenoidal linker protein EPYC1 phase separates with hybrid Arabidopsis-Chlamydomonas Rubisco through interactions with the algal Rubisco small subunit.J Exp Bot. 2019 Oct 15;70(19):5271-5285. doi: 10.1093/jxb/erz275. J Exp Bot. 2019. PMID: 31504763 Free PMC article.
-
Structure and function of LCI1: a plasma membrane CO2 channel in the Chlamydomonas CO2 concentrating mechanism.Plant J. 2020 Jun;102(6):1107-1126. doi: 10.1111/tpj.14745. Epub 2020 Apr 18. Plant J. 2020. PMID: 32168387 Free PMC article.
-
New horizons for building pyrenoid-based CO2-concentrating mechanisms in plants to improve yields.Plant Physiol. 2022 Oct 27;190(3):1609-1627. doi: 10.1093/plphys/kiac373. Plant Physiol. 2022. PMID: 35961043 Free PMC article. Review.
-
Coordination between photorespiration and carbon concentrating mechanism in Chlamydomonas reinhardtii: transcript and protein changes during light-dark diurnal cycles and mixotrophy conditions.Protoplasma. 2019 Jan;256(1):117-130. doi: 10.1007/s00709-018-1283-4. Epub 2018 Jul 10. Protoplasma. 2019. PMID: 29987443
References
-
- Cordat E, Casey JR. Bicarbonate transport in cell physiology and disease. Biochem J. 2009;417(2):423–439. - PubMed
-
- Price GD, Badger MR, Woodger FJ, Long BM. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): Functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J Exp Bot. 2008;59(7):1441–1461. - PubMed
-
- Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature. 2003;425(6959):734–737. - PubMed
-
- Jones HG. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology. 2nd Ed Cambridge Univ Press, Cambridge, UK; 1992.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

