Fast immuno-labeling by electrophoretically driven infiltration for intact tissue imaging
- PMID: 26013317
- PMCID: PMC4603706
- DOI: 10.1038/srep10640
Fast immuno-labeling by electrophoretically driven infiltration for intact tissue imaging
Abstract
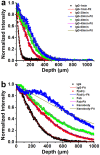
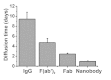
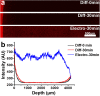
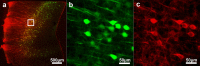
Recently developed tissue clearing techniques, where the tissue is embedded within a hydrogel, have revolutionized our ability to resolve fine cellular structures in nearly intact tissues. However, the slow rate of penetration of antibodies within this hydrogel-tissue matrix has become a significantly limiting factor in many experiments, as thick tissues often require weeks to months to be adequately labeled. Increasing the pore size of this matrix has been investigated as a possible solution, but with only modest success. Here, we have systematically examined the diffusional behavior of antibodies and other typically used immuno-labels within this hydrogel-tissue matrix and, surprisingly, found that infiltration occurs at rates similar to those of diffusion in free solution. Therefore, changing the pore size of the matrix would be expected to afford only limited improvement and, instead, some means of active transport is necessary. We show that an electrophoretically-driven approach decreases the delivery time of antibodies by more than 800-fold over simple diffusion, without incurring structural damage. These results, together with the high quality of the images obtained with this method, demonstrate the advantage of this approach, thus significantly broadening the practical range of samples that can now be investigated by whole-mount tissue clearing methods.
Figures

Similar articles
-
High Definition Confocal Imaging Modalities for the Characterization of Tissue-Engineered Substitutes.Methods Mol Biol. 2018;1773:93-105. doi: 10.1007/978-1-4939-7799-4_8. Methods Mol Biol. 2018. PMID: 29687383
-
Introduction to heavy chain antibodies and derived Nanobodies.Methods Mol Biol. 2012;911:15-26. doi: 10.1007/978-1-61779-968-6_2. Methods Mol Biol. 2012. PMID: 22886243 Review.
-
Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms.Cancer Res. 1992 Jun 15;52(12):3402-8. Cancer Res. 1992. PMID: 1596900
-
Visualizing two-component protein diffusion in porous adsorbents by confocal scanning laser microscopy.Biotechnol Bioeng. 1999 Dec 20;65(6):622-30. Biotechnol Bioeng. 1999. PMID: 10550768
-
Recurrence phenomena after immunoglobulin therapy for snake envenomations: Part 1. Pharmacokinetics and pharmacodynamics of immunoglobulin antivenoms and related antibodies.Ann Emerg Med. 2001 Feb;37(2):189-95. doi: 10.1067/mem.2001.113135. Ann Emerg Med. 2001. PMID: 11174238 Review.
Cited by
-
The role of myelination in measures of white matter integrity: Combination of diffusion tensor imaging and two-photon microscopy of CLARITY intact brains.Neuroimage. 2017 Feb 15;147:253-261. doi: 10.1016/j.neuroimage.2016.11.068. Epub 2016 Dec 13. Neuroimage. 2017. PMID: 27986605 Free PMC article.
-
Comparison of diffusion MRI and CLARITY fiber orientation estimates in both gray and white matter regions of human and primate brain.Neuroimage. 2021 Mar;228:117692. doi: 10.1016/j.neuroimage.2020.117692. Epub 2020 Dec 30. Neuroimage. 2021. PMID: 33385546 Free PMC article.
-
CUBIC pathology: three-dimensional imaging for pathological diagnosis.Sci Rep. 2017 Aug 24;7(1):9269. doi: 10.1038/s41598-017-09117-0. Sci Rep. 2017. PMID: 28839164 Free PMC article.
-
Can Developments in Tissue Optical Clearing Aid Super-Resolution Microscopy Imaging?Int J Mol Sci. 2021 Jun 23;22(13):6730. doi: 10.3390/ijms22136730. Int J Mol Sci. 2021. PMID: 34201632 Free PMC article. Review.
-
Large-scale tissue clearing (PACT): Technical evaluation and new perspectives in immunofluorescence, histology, and ultrastructure.Sci Rep. 2016 Sep 29;6:34331. doi: 10.1038/srep34331. Sci Rep. 2016. PMID: 27680942 Free PMC article.
References
-
- Ertürk A. et al. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nature Protocols 7, 1983–1995 (2012). - PubMed
-
- Ke M., Fujimoto S. & Imai T. SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat. Neurosci. 16, 1154–1161 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

