Toll-Like Receptor Signaling Induces Nrf2 Pathway Activation through p62-Triggered Keap1 Degradation
- PMID: 26012548
- PMCID: PMC4524114
- DOI: 10.1128/MCB.00105-15
Toll-Like Receptor Signaling Induces Nrf2 Pathway Activation through p62-Triggered Keap1 Degradation
Abstract
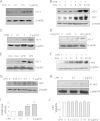
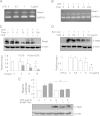
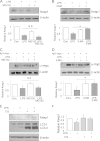
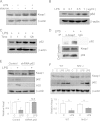
Toll-like receptors (TLRs) induce inflammation and tissue repair through multiple signaling pathways. The Nrf2 pathway plays a key role in defending against the tissue damage incurred by microbial infection or inflammation-associated diseases. The critical event that mediates TLR-induced Nrf2 activation is still poorly understood. In this study, we found that lipopolysaccharide (LPS) and other Toll-like receptor (TLR) agonists activate Nrf2 signaling and the activation is due to the reduction of Keap1, the key Nrf2 inhibitor. TLR signaling-induced Keap1 reduction promoted Nrf2 translocation from the cytoplasm to the nucleus, where it activated transcription of its target genes. TLR agonists modulated Keap1 at the protein posttranslation level through autophagy. TLR signaling increased the expression of autophagy protein p62 and LC3-II and induced their association with Keap1 in the autophagosome-like structures. We also characterized the interaction between p62 and Keap1 and found that p62 is indispensable for TLR-mediated Keap1 reduction: TLR signaling had no effect on Keap1 if cells lacked p62 or if cells expressed a mutant Keap1 that could not interact with p62. Our study indicates that p62-mediated Keap1 degradation through autophagy represents a critical linkage for TLR signaling regulation of the major defense network, the Nrf2 signaling pathway.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1.Nat Cell Biol. 2010 Mar;12(3):213-23. doi: 10.1038/ncb2021. Epub 2010 Feb 21. Nat Cell Biol. 2010. PMID: 20173742
-
p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription.J Biol Chem. 2010 Jul 16;285(29):22576-91. doi: 10.1074/jbc.M110.118976. Epub 2010 May 7. J Biol Chem. 2010. PMID: 20452972 Free PMC article.
-
PF-4708671, a specific inhibitor of p70 ribosomal S6 kinase 1, activates Nrf2 by promoting p62-dependent autophagic degradation of Keap1.Biochem Biophys Res Commun. 2015 Oct 23;466(3):499-504. doi: 10.1016/j.bbrc.2015.09.059. Epub 2015 Sep 14. Biochem Biophys Res Commun. 2015. PMID: 26381178
-
Novel target for treating Alzheimer's Diseases: Crosstalk between the Nrf2 pathway and autophagy.Ageing Res Rev. 2021 Jan;65:101207. doi: 10.1016/j.arr.2020.101207. Epub 2020 Nov 1. Ageing Res Rev. 2021. PMID: 33144123 Review.
-
p62/SQSTM1 functions as a signaling hub and an autophagy adaptor.FEBS J. 2015 Dec;282(24):4672-8. doi: 10.1111/febs.13540. Epub 2015 Oct 16. FEBS J. 2015. PMID: 26432171 Review.
Cited by
-
Polarization of Low-Grade Inflammatory Monocytes Through TRAM-Mediated Up-Regulation of Keap1 by Super-Low Dose Endotoxin.Front Immunol. 2020 Jul 16;11:1478. doi: 10.3389/fimmu.2020.01478. eCollection 2020. Front Immunol. 2020. PMID: 32765513 Free PMC article.
-
Clinical implication of Keap1 and phosphorylated Nrf2 expression in hepatocellular carcinoma.Cancer Med. 2016 Oct;5(10):2678-2687. doi: 10.1002/cam4.788. Epub 2016 Sep 20. Cancer Med. 2016. PMID: 27650414 Free PMC article.
-
The different roles of selective autophagic protein degradation in mammalian cells.Oncotarget. 2015 Nov 10;6(35):37098-116. doi: 10.18632/oncotarget.5776. Oncotarget. 2015. PMID: 26415220 Free PMC article. Review.
-
Potential Applications of NRF2 Inhibitors in Cancer Therapy.Oxid Med Cell Longev. 2019 Apr 11;2019:8592348. doi: 10.1155/2019/8592348. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31097977 Free PMC article. Review.
-
TGF-β induces miR-30d down-regulation and podocyte injury through Smad2/3 and HDAC3-associated transcriptional repression.J Mol Med (Berl). 2016 Mar;94(3):291-300. doi: 10.1007/s00109-015-1340-9. Epub 2015 Oct 3. J Mol Med (Berl). 2016. PMID: 26432290
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
