Two-pore channels at the intersection of endolysosomal membrane traffic
- PMID: 26009187
- PMCID: PMC4730950
- DOI: 10.1042/BST20140303
Two-pore channels at the intersection of endolysosomal membrane traffic
Abstract
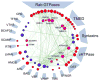
Two-pore channels (TPCs) are ancient members of the voltage-gated ion channel superfamily that localize to acidic organelles such as lysosomes. The TPC complex is the proposed target of the Ca2+-mobilizing messenger NAADP, which releases Ca2+ from these acidic Ca2+ stores. Whereas details of TPC activation and native ion permeation remain unclear, a consensus has emerged around their function in regulating endolysosomal trafficking. This role is supported by recent proteomic data showing that TPCs interact with proteins controlling membrane organization and dynamics, including Rab GTPases and components of the fusion apparatus. Regulation of TPCs by PtdIns(3,5)P2 and/or NAADP (nicotinic acid adenine dinucleotide phosphate) together with their functional and physical association with Rab proteins provides a mechanism for coupling phosphoinositide and trafficking protein cues to local ion fluxes. Therefore, TPCs work at the regulatory cross-roads of (patho)physiological cues to co-ordinate and potentially deregulate traffic flow through the endolysosomal network. This review focuses on the native role of TPCs in trafficking and their emerging contributions to endolysosomal trafficking dysfunction.
Figures
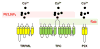
Similar articles
-
Function and dysfunction of two-pore channels.Sci Signal. 2015 Jul 7;8(384):re7. doi: 10.1126/scisignal.aab3314. Sci Signal. 2015. PMID: 26152696 Review.
-
The Two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation.Proc Natl Acad Sci U S A. 2014 Sep 9;111(36):13087-92. doi: 10.1073/pnas.1407004111. Epub 2014 Aug 25. Proc Natl Acad Sci U S A. 2014. PMID: 25157141 Free PMC article.
-
TPCs: Endolysosomal channels for Ca2+ mobilization from acidic organelles triggered by NAADP.FEBS Lett. 2010 May 17;584(10):1966-74. doi: 10.1016/j.febslet.2010.02.028. Epub 2010 Feb 14. FEBS Lett. 2010. PMID: 20159015 Free PMC article. Review.
-
TPC: the NAADP discovery channel?Biochem Soc Trans. 2015 Jun;43(3):384-9. doi: 10.1042/BST20140300. Biochem Soc Trans. 2015. PMID: 26009180 Review.
-
NAADP as an intracellular messenger regulating lysosomal calcium-release channels.Biochem Soc Trans. 2010 Dec;38(6):1424-31. doi: 10.1042/BST0381424. Biochem Soc Trans. 2010. PMID: 21118101
Cited by
-
The ins and outs of virus trafficking through acidic Ca2+ stores.Cell Calcium. 2022 Mar;102:102528. doi: 10.1016/j.ceca.2022.102528. Epub 2022 Jan 6. Cell Calcium. 2022. PMID: 35033909 Free PMC article. Review.
-
Ion channels in the regulation of autophagy.Autophagy. 2018;14(1):3-21. doi: 10.1080/15548627.2017.1384887. Epub 2017 Nov 23. Autophagy. 2018. PMID: 28980859 Free PMC article. Review.
-
NAADP-dependent Ca2+ signaling regulates Middle East respiratory syndrome-coronavirus pseudovirus translocation through the endolysosomal system.Cell Calcium. 2018 Nov;75:30-41. doi: 10.1016/j.ceca.2018.08.003. Epub 2018 Aug 9. Cell Calcium. 2018. PMID: 30121440 Free PMC article.
-
Electrophysiology of Endolysosomal Two-Pore Channels: A Current Account.Cells. 2022 Aug 2;11(15):2368. doi: 10.3390/cells11152368. Cells. 2022. PMID: 35954212 Free PMC article. Review.
-
Two-pore channels affect EGF receptor signaling by receptor trafficking and expression.iScience. 2021 Jan 27;24(2):102099. doi: 10.1016/j.isci.2021.102099. eCollection 2021 Feb 19. iScience. 2021. PMID: 33644717 Free PMC article.
References
-
- Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10:623–635. - PubMed
-
- Ishibashi K, Suzuki M, Imai M. Molecular cloning of a novel form (two-repeat) protein related to voltage-gated sodium and calcium channels. Biochem Biophys Res Commun. 2000;270:370–376. - PubMed
-
- Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev. 2005;57:387–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

