Lymph node fibroblastic reticular cells in health and disease
- PMID: 25998961
- PMCID: PMC5152733
- DOI: 10.1038/nri3846
Lymph node fibroblastic reticular cells in health and disease
Abstract
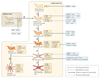
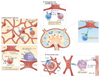
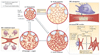
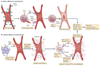
Over the past decade, a series of discoveries relating to fibroblastic reticular cells (FRCs) — immunologically specialized myofibroblasts found in lymphoid tissue — has promoted these cells from benign bystanders to major players in the immune response. In this Review, we focus on recent advances regarding the immunobiology of lymph node-derived FRCs, presenting an updated view of crucial checkpoints during their development and their dynamic control of lymph node expansion and contraction during infection. We highlight the robust effects of FRCs on systemic B cell and T cell responses, and we present an emerging view of FRCs as drivers of pathology following acute and chronic viral infections. Lastly, we review emerging therapeutic advances that harness the immunoregulatory properties of FRCs.
Conflict of interest statement
statement A.L.F. is an inventor on a patent related to the therapeutic use of FRCs.
Figures
Similar articles
-
Characterization of human fibroblastic reticular cells as potential immunotherapeutic tools.Cytotherapy. 2017 May;19(5):640-653. doi: 10.1016/j.jcyt.2017.01.010. Epub 2017 Mar 2. Cytotherapy. 2017. PMID: 28262465
-
Gremlin 1+ fibroblastic niche maintains dendritic cell homeostasis in lymphoid tissues.Nat Immunol. 2021 May;22(5):571-585. doi: 10.1038/s41590-021-00920-6. Epub 2021 Apr 26. Nat Immunol. 2021. PMID: 33903764
-
Maturation of lymph node fibroblastic reticular cells from myofibroblastic precursors is critical for antiviral immunity.Immunity. 2013 May 23;38(5):1013-24. doi: 10.1016/j.immuni.2013.03.012. Epub 2013 Apr 25. Immunity. 2013. PMID: 23623380 Free PMC article.
-
Guiding blind T cells and dendritic cells: A closer look at fibroblastic reticular cells found within lymph node T zones.Immunol Lett. 2011 Jul;138(1):9-11. doi: 10.1016/j.imlet.2011.02.006. Epub 2011 Feb 17. Immunol Lett. 2011. PMID: 21333683 Review.
-
Fibroblastic reticular cells: organization and regulation of the T lymphocyte life cycle.J Immunol. 2015 Feb 15;194(4):1389-94. doi: 10.4049/jimmunol.1402520. J Immunol. 2015. PMID: 25663676 Free PMC article. Review.
Cited by
-
Bioengineering of Artificial Lymphoid Organs.Acta Naturae. 2016 Apr-Jun;8(2):10-23. Acta Naturae. 2016. PMID: 27437136 Free PMC article.
-
The Multifaceted Effects of Breast Cancer on Tumor-Draining Lymph Nodes.Am J Pathol. 2021 Aug;191(8):1353-1363. doi: 10.1016/j.ajpath.2021.05.006. Epub 2021 May 24. Am J Pathol. 2021. PMID: 34043978 Free PMC article. Review.
-
Modeling the crosstalk between malignant B cells and their microenvironment in B-cell lymphomas: challenges and opportunities.Front Immunol. 2023 Nov 2;14:1288110. doi: 10.3389/fimmu.2023.1288110. eCollection 2023. Front Immunol. 2023. PMID: 38022603 Free PMC article. Review.
-
Clinical, histopathologic, and immunoarchitectural features of dermatopathic lymphadenopathy: an update.Mod Pathol. 2020 Jun;33(6):1104-1121. doi: 10.1038/s41379-019-0440-4. Epub 2020 Jan 2. Mod Pathol. 2020. PMID: 31896812
-
Targeting Discoidin Domain Receptors DDR1 and DDR2 overcomes matrix-mediated tumor cell adaptation and tolerance to BRAF-targeted therapy in melanoma.EMBO Mol Med. 2022 Feb 7;14(2):e11814. doi: 10.15252/emmm.201911814. Epub 2021 Dec 27. EMBO Mol Med. 2022. PMID: 34957688 Free PMC article.
References
-
- Link A, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

