Turning Saccharomyces cerevisiae into a Frataxin-Independent Organism
- PMID: 25996596
- PMCID: PMC4440810
- DOI: 10.1371/journal.pgen.1005135
Turning Saccharomyces cerevisiae into a Frataxin-Independent Organism
Abstract
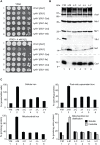
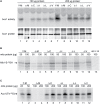
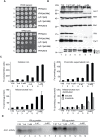
Frataxin (Yfh1 in yeast) is a conserved protein and deficiency leads to the neurodegenerative disease Friedreich's ataxia. Frataxin is a critical protein for Fe-S cluster assembly in mitochondria, interacting with other components of the Fe-S cluster machinery, including cysteine desulfurase Nfs1, Isd11 and the Isu1 scaffold protein. Yeast Isu1 with the methionine to isoleucine substitution (M141I), in which the E. coli amino acid is inserted at this position, corrected most of the phenotypes that result from lack of Yfh1 in yeast. This suppressor Isu1 behaved as a genetic dominant. Furthermore frataxin-bypass activity required a completely functional Nfs1 and correlated with the presence of efficient scaffold function. A screen of random Isu1 mutations for frataxin-bypass activity identified only M141 substitutions, including Ile, Cys, Leu, or Val. In each case, mitochondrial Nfs1 persulfide formation was enhanced, and mitochondrial Fe-S cluster assembly was improved in the absence of frataxin. Direct targeting of the entire E. coli IscU to ∆yfh1 mitochondria also ameliorated the mutant phenotypes. In contrast, expression of IscU with the reverse substitution i.e. IscU with Ile to Met change led to worsening of the ∆yfh1 phenotypes, including severely compromised growth, increased sensitivity to oxygen, deficiency in Fe-S clusters and heme, and impaired iron homeostasis. A bioinformatic survey of eukaryotic Isu1/prokaryotic IscU database entries sorted on the amino acid utilized at the M141 position identified unique groupings, with virtually all of the eukaryotic scaffolds using Met, and the preponderance of prokaryotic scaffolds using other amino acids. The frataxin-bypassing amino acids Cys, Ile, Leu, or Val, were found predominantly in prokaryotes. This amino acid position 141 is unique in Isu1, and the frataxin-bypass effect likely mimics a conserved and ancient feature of the prokaryotic Fe-S cluster assembly machinery.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

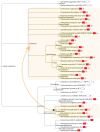
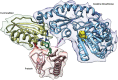
Comment in
-
Trading Places-Switching Frataxin Function by a Single Amino Acid Substitution within the [Fe-S] Cluster Assembly Scaffold.PLoS Genet. 2015 May 21;11(5):e1005192. doi: 10.1371/journal.pgen.1005192. eCollection 2015 May. PLoS Genet. 2015. PMID: 25996679 Free PMC article. No abstract available.
Similar articles
-
Frataxin directly stimulates mitochondrial cysteine desulfurase by exposing substrate-binding sites, and a mutant Fe-S cluster scaffold protein with frataxin-bypassing ability acts similarly.J Biol Chem. 2013 Dec 27;288(52):36773-86. doi: 10.1074/jbc.M113.525857. Epub 2013 Nov 11. J Biol Chem. 2013. PMID: 24217246 Free PMC article.
-
Mutation in the Fe-S scaffold protein Isu bypasses frataxin deletion.Biochem J. 2012 Jan 1;441(1):473-80. doi: 10.1042/BJ20111637. Biochem J. 2012. PMID: 21936771 Free PMC article.
-
Frataxin-bypassing Isu1: characterization of the bypass activity in cells and mitochondria.Biochem J. 2014 Apr 1;459(1):71-81. doi: 10.1042/BJ20131273. Biochem J. 2014. PMID: 24433162 Free PMC article.
-
The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism.Biochim Biophys Acta. 2012 Sep;1823(9):1491-508. doi: 10.1016/j.bbamcr.2012.05.009. Epub 2012 May 15. Biochim Biophys Acta. 2012. PMID: 22609301 Review.
-
Molecular Details of the Frataxin-Scaffold Interaction during Mitochondrial Fe-S Cluster Assembly.Int J Mol Sci. 2021 Jun 2;22(11):6006. doi: 10.3390/ijms22116006. Int J Mol Sci. 2021. PMID: 34199378 Free PMC article. Review.
Cited by
-
Characteristics of the Isu1 C-terminus in relation to [2Fe-2S] cluster assembly and ISCU Myopathy.J Biol Inorg Chem. 2022 Dec;27(8):759-773. doi: 10.1007/s00775-022-01964-1. Epub 2022 Oct 30. J Biol Inorg Chem. 2022. PMID: 36309885
-
In vitro characterization of a novel Isu homologue from Drosophila melanogaster for de novo FeS-cluster formation.Metallomics. 2017 Jan 25;9(1):48-60. doi: 10.1039/c6mt00163g. Metallomics. 2017. PMID: 27738674 Free PMC article.
-
N-terminal tyrosine of ISCU2 triggers [2Fe-2S] cluster synthesis by ISCU2 dimerization.Nat Commun. 2021 Nov 25;12(1):6902. doi: 10.1038/s41467-021-27122-w. Nat Commun. 2021. PMID: 34824239 Free PMC article.
-
Trading Places-Switching Frataxin Function by a Single Amino Acid Substitution within the [Fe-S] Cluster Assembly Scaffold.PLoS Genet. 2015 May 21;11(5):e1005192. doi: 10.1371/journal.pgen.1005192. eCollection 2015 May. PLoS Genet. 2015. PMID: 25996679 Free PMC article. No abstract available.
-
Crystal Structure of Bacillus subtilis Cysteine Desulfurase SufS and Its Dynamic Interaction with Frataxin and Scaffold Protein SufU.PLoS One. 2016 Jul 6;11(7):e0158749. doi: 10.1371/journal.pone.0158749. eCollection 2016. PLoS One. 2016. PMID: 27382962 Free PMC article.
References
-
- Gibson TJ, Koonin EV, Musco G, Pastore A, Bork P (1996) Friedreich's ataxia protein: phylogenetic evidence for mitochondrial dysfunction. Trends Neurosci 19: 465–468. - PubMed
-
- Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, Cavalcanti F, et al. (1996) Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271: 1423–1427. - PubMed
-
- Schmucker S, Martelli A, Colin F, Page A, Wattenhofer-Donze M, Reutenauer L, et al. (2011) Mammalian frataxin: an essential function for cellular viability through an interaction with a preformed ISCU/NFS1/ISD11 iron-sulfur assembly complex. PLoS One 6: e16199 10.1371/journal.pone.0016199 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

