The sexual dimorphism associated with pulmonary hypertension corresponds to a fibrotic phenotype
- PMID: 25992281
- PMCID: PMC4405725
- DOI: 10.1086/679724
The sexual dimorphism associated with pulmonary hypertension corresponds to a fibrotic phenotype
Abstract
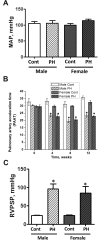
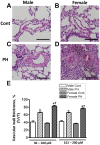
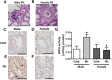
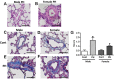
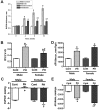
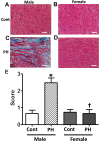
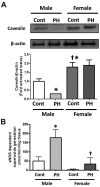
Although female predominance in the development of all types of pulmonary hypertension (PH) is well established, many clinical studies have confirmed that females have better prognosis and higher survival rate than males. There is no clear explanation of why sex influences the pathogenesis and progression of PH. Using a rat angioproliferative model of PH, which closely resembles the primary pathological changes observed in humans, we evaluated the role of sex in the development and progression of PH. Female rats had a more pronounced increase in medial thickness in the small pulmonary arteries. However, the infiltration of small pulmonary arteries by inflammatory cells was found only in male rats, and this corresponded to increased myeloperoxidase activity and abundant adventitial and medial fibrosis that were not present in female rats. Although the level of right ventricle (RV) peak systolic pressure was similar in both groups, the survival rate in male rats was significantly lower. Moreover, male rats presented with a more pronounced increase in RV thickness that correlated with diffuse RV fibrosis and significantly impaired right cardiac function. The reduction in fibrosis in female rats correlated with increased expression of caveolin-1 and reduced endothelial nitric oxide synthase-derived superoxide. We conclude that, in the pathogenesis of PH, female sex is associated with greater remodeling of the pulmonary arteries but greater survival. Conversely, in males, the development of pulmonary and cardiac fibrosis leads to early and severe RV failure, and this may be an important reason for the lower survival rate among males.
Keywords: caveolin-1; nitric oxide synthase; oxidative stress; superoxide.
Figures

Similar articles
-
Role of endothelial nitric oxide synthase and collagen metabolism in right ventricular remodeling due to pulmonary hypertension.Circ J. 2014;78(6):1465-74. doi: 10.1253/circj.cj-13-1586. Epub 2014 Apr 4. Circ J. 2014. PMID: 24705390
-
ASK1 Inhibition Halts Disease Progression in Preclinical Models of Pulmonary Arterial Hypertension.Am J Respir Crit Care Med. 2018 Feb 1;197(3):373-385. doi: 10.1164/rccm.201703-0502OC. Am J Respir Crit Care Med. 2018. PMID: 28910144
-
Pharmacological Inhibition of mTOR Kinase Reverses Right Ventricle Remodeling and Improves Right Ventricle Structure and Function in Rats.Am J Respir Cell Mol Biol. 2017 Nov;57(5):615-625. doi: 10.1165/rcmb.2016-0364OC. Am J Respir Cell Mol Biol. 2017. PMID: 28679058 Free PMC article.
-
The HMG-CoA reductase inhibitor, pravastatin, prevents the development of monocrotaline-induced pulmonary hypertension in the rat through reduction of endothelial cell apoptosis and overexpression of eNOS.Naunyn Schmiedebergs Arch Pharmacol. 2006 Sep;373(6):401-14. doi: 10.1007/s00210-006-0082-1. Epub 2006 Aug 1. Naunyn Schmiedebergs Arch Pharmacol. 2006. PMID: 16896805
-
The right ventricle and pulmonary hypertension.Heart Fail Rev. 2016 May;21(3):259-71. doi: 10.1007/s10741-016-9526-y. Heart Fail Rev. 2016. PMID: 26833318 Review.
Cited by
-
Role of Gender in Regulation of Redox Homeostasis in Pulmonary Arterial Hypertension.Antioxidants (Basel). 2019 May 16;8(5):135. doi: 10.3390/antiox8050135. Antioxidants (Basel). 2019. PMID: 31100969 Free PMC article. Review.
-
Sirtuin 3 and Uncouplin Protein 2, the Missing Link Between Genetics, Metabolism, and Pulmonary Arterial Hypertension.J Am Heart Assoc. 2021 Dec 7;10(23):e023065. doi: 10.1161/JAHA.121.023065. Epub 2021 Nov 2. J Am Heart Assoc. 2021. PMID: 34724803 Free PMC article. No abstract available.
-
Sex-specific stress response and HMGB1 release in pulmonary endothelial cells.PLoS One. 2020 Apr 9;15(4):e0231267. doi: 10.1371/journal.pone.0231267. eCollection 2020. PLoS One. 2020. PMID: 32271800 Free PMC article.
-
Recurrent inhibition of mitochondrial complex III induces chronic pulmonary vasoconstriction and glycolytic switch in the rat lung.Respir Res. 2018 Apr 23;19(1):69. doi: 10.1186/s12931-018-0776-1. Respir Res. 2018. PMID: 29685148 Free PMC article.
-
Apoptosis signal-regulating kinase 1 inhibition in in vivo and in vitro models of pulmonary hypertension.Pulm Circ. 2020 May 18;10(2):2045894020922810. doi: 10.1177/2045894020922810. eCollection 2020 Apr-Jun. Pulm Circ. 2020. PMID: 32523684 Free PMC article.
References
-
- Runo JR, Loyd JE. Primary pulmonary hypertension. Lancet 2003;361:1533–1544. - PubMed
-
- Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension: a national prospective study. Ann Intern Med 1987;107:216–223. - PubMed
-
- Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL registry. Chest 2010;137:376–387. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

