Vaccination with Melanoma Helper Peptides Induces Antibody Responses Associated with Improved Overall Survival
- PMID: 25967144
- PMCID: PMC4558239
- DOI: 10.1158/1078-0432.CCR-15-0233
Vaccination with Melanoma Helper Peptides Induces Antibody Responses Associated with Improved Overall Survival
Abstract
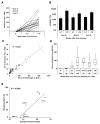
Purpose: A melanoma vaccine incorporating six peptides designed to induce helper T-cell responses to melanoma antigens has induced Th1-dominant CD4(+) T-cell responses in most patients, and induced durable clinical responses or stable disease in 24% of evaluable patients. The present study tested whether this vaccine also induced antibody (Ab) responses to each peptide, and whether Ab responses were associated with T-cell responses and with clinical outcome.
Experimental design: Serum samples were studied from 35 patients with stage III-IV melanomas vaccinated with 6 melanoma helper peptides (6MHP). IgG Ab responses were measured by ELISA. Associations with immune response and overall survival were assessed by log-rank test and χ(2) analysis of Kaplan-Meier data.
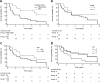
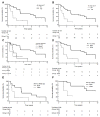
Results: Ab responses to 6MHP were detected by week 7 in 77% of patients, and increased to peak 6 weeks after the last vaccine and persisted to 6 months. Ab responses were induced most frequently to longer peptides. Of those with T-cell responses, 82% had early Ab responses. Survival was improved for patients with early Ab response (P = 0.0011) or with early T-cell response (P < 0.006), and was best for those with both Ab and T-cell responses (P = 0.0002).
Conclusions: Vaccination with helper peptides induced both Ab responses and T-cell responses, associated with favorable clinical outcome. Such immune responses may predict favorable clinical outcome to guide combination immunotherapy. Further studies are warranted to understand mechanisms of interaction of these Abs, T-cell responses, and tumor control.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures
Similar articles
-
Randomized multicenter trial of the effects of melanoma-associated helper peptides and cyclophosphamide on the immunogenicity of a multipeptide melanoma vaccine.J Clin Oncol. 2011 Jul 20;29(21):2924-32. doi: 10.1200/JCO.2010.33.8053. Epub 2011 Jun 20. J Clin Oncol. 2011. PMID: 21690475 Free PMC article. Clinical Trial.
-
Long-term outcomes of helper peptide vaccination for metastatic melanoma.Ann Surg. 2015 Sep;262(3):456-64; discussion 462-4. doi: 10.1097/SLA.0000000000001419. Ann Surg. 2015. PMID: 26258314 Free PMC article. Clinical Trial.
-
A randomized phase II trial of multiepitope vaccination with melanoma peptides for cytotoxic T cells and helper T cells for patients with metastatic melanoma (E1602).Clin Cancer Res. 2013 Aug 1;19(15):4228-38. doi: 10.1158/1078-0432.CCR-13-0002. Epub 2013 May 7. Clin Cancer Res. 2013. PMID: 23653149 Free PMC article. Clinical Trial.
-
Trial to evaluate the immunogenicity and safety of a melanoma helper peptide vaccine plus incomplete Freund's adjuvant, cyclophosphamide, and polyICLC (Mel63).J Immunother Cancer. 2021 Jan;9(1):e000934. doi: 10.1136/jitc-2020-000934. J Immunother Cancer. 2021. PMID: 33479025 Free PMC article. Clinical Trial.
-
Immune modulations during chemoimmunotherapy & novel vaccine strategies--in metastatic melanoma and non small-cell lung cancer.Dan Med J. 2013 Dec;60(12):B4774. Dan Med J. 2013. PMID: 24355457 Review.
Cited by
-
A randomized pilot trial testing the safety and immunologic effects of a MAGE-A3 protein plus AS15 immunostimulant administered into muscle or into dermal/subcutaneous sites.Cancer Immunol Immunother. 2016 Jan;65(1):25-36. doi: 10.1007/s00262-015-1770-9. Epub 2015 Nov 18. Cancer Immunol Immunother. 2016. PMID: 26581199 Free PMC article. Clinical Trial.
-
PSGL-1 Immune Checkpoint Inhibition for CD4+ T Cell Cancer Immunotherapy.Front Immunol. 2021 Feb 23;12:636238. doi: 10.3389/fimmu.2021.636238. eCollection 2021. Front Immunol. 2021. PMID: 33708224 Free PMC article. Review.
-
Phase I/II clinical trial of a helper peptide vaccine plus PD-1 blockade in PD-1 antibody-naïve and PD-1 antibody-experienced patients with melanoma (MEL64).J Immunother Cancer. 2022 Sep;10(9):e005424. doi: 10.1136/jitc-2022-005424. J Immunother Cancer. 2022. PMID: 36100309 Free PMC article. Clinical Trial.
-
Peptide emulsions in incomplete Freund's adjuvant create effective nurseries promoting egress of systemic CD4+ and CD8+ T cells for immunotherapy of cancer.J Immunother Cancer. 2022 Sep;10(9):e004709. doi: 10.1136/jitc-2022-004709. J Immunother Cancer. 2022. PMID: 36939214 Free PMC article. Review.
-
Updates in adjuvant systemic therapy for melanoma.J Surg Oncol. 2019 Jan;119(2):222-231. doi: 10.1002/jso.25298. Epub 2018 Nov 27. J Surg Oncol. 2019. PMID: 30481375 Free PMC article. Review.
References
-
- Swartz A, Batich K, Fecci P, Sampson J. Peptide vaccines for the treatment of glioblastoma. J Neurooncol. 2014:1–8. - PubMed
-
- Kobold S, Lütkens T, Cao Y, Bokemeyer C, Atanackovic D. Autoantibodies against tumor-related antigens: Incidence and biologic significance. Human Immunology. 2010;71:643–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

