Reactive astrocytes as neural stem or progenitor cells: In vivo lineage, In vitro potential, and Genome-wide expression analysis
- PMID: 25965557
- PMCID: PMC5029574
- DOI: 10.1002/glia.22850
Reactive astrocytes as neural stem or progenitor cells: In vivo lineage, In vitro potential, and Genome-wide expression analysis
Abstract
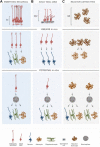
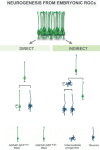
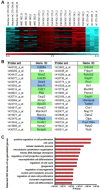
Here, we review the stem cell hallmarks of endogenous neural stem cells (NSCs) during development and in some niches of the adult mammalian brain to then compare these with reactive astrocytes acquiring stem cell hallmarks after traumatic and ischemic brain injury. Notably, even endogenous NSCs including the earliest NSCs, the neuroepithelial cells, generate in most cases only a single type of progeny and self-renew only for a rather short time in vivo. In vitro, however, especially cells cultured under neurosphere conditions reveal a larger potential and long-term self-renewal under the influence of growth factors. This is rather well comparable to reactive astrocytes in the traumatic or ischemic brain some of which acquire neurosphere-forming capacity including multipotency and long-term self-renewal in vitro, while they remain within their astrocyte lineage in vivo. Both reactive astrocytes and endogenous NSCs exhibit stem cell hallmarks largely in vitro, but their lineage differs in vivo. Both populations generate largely a single cell type in vivo, but endogenous NSCs generate neurons and reactive astrocytes remain in the astrocyte lineage. However, at some early postnatal stages or in some brain regions reactive astrocytes can be released from this fate restriction, demonstrating that they can also enact neurogenesis. Thus, reactive astrocytes and NSCs share many characteristic hallmarks, but also exhibit key differences. This conclusion is further substantiated by genome-wide expression analysis comparing NSCs at different stages with astrocytes from the intact and injured brain parenchyma.
Keywords: brain injury; lineage; potential; radial glial cells; self-renewal; transcriptome.
© 2015 The Authors. Glia Published by Wiley Periodicals, Inc.
Figures
Similar articles
-
Self-renewal and differentiation of reactive astrocyte-derived neural stem/progenitor cells isolated from the cortical peri-infarct area after stroke.J Neurosci. 2012 Jun 6;32(23):7926-40. doi: 10.1523/JNEUROSCI.4303-11.2012. J Neurosci. 2012. PMID: 22674268 Free PMC article.
-
The Neurogenic Potential of Astrocytes Is Regulated by Inflammatory Signals.Mol Neurobiol. 2016 Aug;53(6):3724-3739. doi: 10.1007/s12035-015-9296-x. Epub 2015 Jul 4. Mol Neurobiol. 2016. PMID: 26138449 Free PMC article.
-
Neural stem cells transplanted into intact brains as neurospheres form solid grafts composed of neurons, astrocytes and oligodendrocyte precursors.Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004 Dec;148(2):217-20. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004. PMID: 15744380
-
Role of Astrocytes in the Neurogenic Niches.Methods Mol Biol. 2019;1938:19-33. doi: 10.1007/978-1-4939-9068-9_2. Methods Mol Biol. 2019. PMID: 30617970 Review.
-
Radial stem astrocytes (aka neural stem cells): Identity, development, physio-pathology, and therapeutic potential.Acta Physiol (Oxf). 2023 Jun;238(2):e13967. doi: 10.1111/apha.13967. Epub 2023 Apr 7. Acta Physiol (Oxf). 2023. PMID: 36971751 Review.
Cited by
-
Identification of Radial Glia Progenitors in the Developing and Adult Retina of Sharks.Front Neuroanat. 2016 Jun 20;10:65. doi: 10.3389/fnana.2016.00065. eCollection 2016. Front Neuroanat. 2016. PMID: 27378863 Free PMC article.
-
Astrocyte reactivity after brain injury-: The role of galectins 1 and 3.Glia. 2015 Dec;63(12):2340-61. doi: 10.1002/glia.22898. Epub 2015 Aug 6. Glia. 2015. PMID: 26250529 Free PMC article.
-
Astrocyte Differentiation of Human Pluripotent Stem Cells: New Tools for Neurological Disorder Research.Front Cell Neurosci. 2016 Sep 26;10:215. doi: 10.3389/fncel.2016.00215. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27725795 Free PMC article. Review.
-
Protective Effects of Early Caffeine Administration in Hyperoxia-Induced Neurotoxicity in the Juvenile Rat.Antioxidants (Basel). 2023 Jan 28;12(2):295. doi: 10.3390/antiox12020295. Antioxidants (Basel). 2023. PMID: 36829854 Free PMC article.
-
Adult neurogenesis from reprogrammed astrocytes.Neural Regen Res. 2020 Jun;15(6):973-979. doi: 10.4103/1673-5374.270292. Neural Regen Res. 2020. PMID: 31823866 Free PMC article. Review.
References
-
- Amat JA, Ishiguro H, Nakamura K, Norton WT. 1996. Phenotypic diversity and kinetics of proliferating microglia and astrocytes following cortical stab wounds. Glia 16:368–382. - PubMed
-
- Anderson SA, Kaznowski CE, Horn C, Rubenstein JL, McConnell SK. 2002. Distinct origins of neocortical projection neurons and interneurons in vivo. Cereb Cortex 12:702–709. - PubMed
-
- Arendt T. 2012. Cell cycle activation and aneuploid neurons in Alzheimer's disease. Mol Neurobiol 46:125–135. - PubMed
-
- Bardehle S, Krüger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer‐Luehmann M, Bechmann I, Dimou L, Götz M. 2013. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci 16:580–586. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

