Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism
- PMID: 25964487
- PMCID: PMC4458459
- DOI: 10.4049/jimmunol.1402469
Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism
Abstract
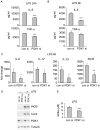
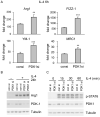
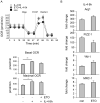
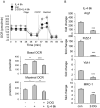
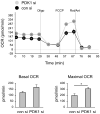
The M1 and M2 polarized phenotypes dictate distinctive roles for macrophages as they participate in inflammatory disorders. There has been growing interest in the role of cellular metabolism in macrophage polarization. However, it is currently unclear whether different aspects of a specific metabolic program coordinately regulate this cellular process. In this study, we found that pyruvate dehydrogenase kinase 1 (PDK1), a key regulatory enzyme in glucose metabolism, plays an important role in the differential activation of macrophages. Knockdown of PDK1 diminished M1, whereas it enhanced M2 activation of macrophages. Mechanistically, PDK1 knockdown led to diminished aerobic glycolysis in M1 macrophages, which likely accounts for the attenuated inflammatory response in these cells. Furthermore, we found that mitochondrial respiration is enhanced during and required by the early activation of M2 macrophages. Suppression of glucose oxidation, but not that of fatty acids, inhibits this process. Consistent with its inhibitory role in early M2 activation, knockdown of PDK1 enhanced mitochondrial respiration in macrophages. Our data suggest that two arms of the glucose metabolism synergistically regulate the differential activation of macrophages. Our findings also highlight the central role of PDK1 in this event via controlling glycolysis and glucose oxidation.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures



Similar articles
-
Vibrio cholerae porin OmpU mediates M1-polarization of macrophages/monocytes via TLR1/TLR2 activation.Immunobiology. 2015 Nov;220(11):1199-209. doi: 10.1016/j.imbio.2015.06.009. Epub 2015 Jun 5. Immunobiology. 2015. PMID: 26093918
-
Pro-inflammatory Macrophages Sustain Pyruvate Oxidation through Pyruvate Dehydrogenase for the Synthesis of Itaconate and to Enable Cytokine Expression.J Biol Chem. 2016 Feb 19;291(8):3932-46. doi: 10.1074/jbc.M115.676817. Epub 2015 Dec 17. J Biol Chem. 2016. PMID: 26679997 Free PMC article.
-
Autocrine IL-10 functions as a rheostat for M1 macrophage glycolytic commitment by tuning nitric oxide production.Redox Biol. 2016 Dec;10:12-23. doi: 10.1016/j.redox.2016.09.005. Epub 2016 Sep 16. Redox Biol. 2016. PMID: 27676159 Free PMC article.
-
Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal.Eur J Immunol. 2016 Jan;46(1):13-21. doi: 10.1002/eji.201445427. Eur J Immunol. 2016. PMID: 26643360 Review.
-
Macrophage Immunometabolism: Where Are We (Going)?Trends Immunol. 2017 Jun;38(6):395-406. doi: 10.1016/j.it.2017.03.001. Epub 2017 Apr 7. Trends Immunol. 2017. PMID: 28396078 Review.
Cited by
-
Loss of metabolic flexibility as a result of overexpression of pyruvate dehydrogenase kinases in muscle, liver and the immune system: Therapeutic targets in metabolic diseases.J Diabetes Investig. 2021 Jan;12(1):21-31. doi: 10.1111/jdi.13345. Epub 2020 Sep 10. J Diabetes Investig. 2021. PMID: 32628351 Free PMC article. Review.
-
Embryonic Origin and Subclonal Evolution of Tumor-Associated Macrophages Imply Preventive Care for Cancer.Cells. 2021 Apr 14;10(4):903. doi: 10.3390/cells10040903. Cells. 2021. PMID: 33919979 Free PMC article. Review.
-
Pyruvate dehydrogenase kinase is a negative regulator of interleukin-10 production in macrophages.J Mol Cell Biol. 2020 Jul 1;12(7):543-555. doi: 10.1093/jmcb/mjz113. J Mol Cell Biol. 2020. PMID: 31900478 Free PMC article.
-
Neuroimmunometabolism: A New Pathological Nexus Underlying Neurodegenerative Disorders.J Neurosci. 2022 Mar 9;42(10):1888-1907. doi: 10.1523/JNEUROSCI.0998-21.2022. Epub 2022 Jan 13. J Neurosci. 2022. PMID: 35027409 Free PMC article. Review.
-
Immunometabolism: Cellular Metabolism Turns Immune Regulator.J Biol Chem. 2016 Jan 1;291(1):1-10. doi: 10.1074/jbc.R115.693903. Epub 2015 Nov 3. J Biol Chem. 2016. PMID: 26534957 Free PMC article. Review.
References
-
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. - PubMed
-
- Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. - PubMed
-
- Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

