Nitric oxide-releasing poly(lactic-co-glycolic acid)-polyethylenimine nanoparticles for prolonged nitric oxide release, antibacterial efficacy, and in vivo wound healing activity
- PMID: 25960648
- PMCID: PMC4411019
- DOI: 10.2147/IJN.S82199
Nitric oxide-releasing poly(lactic-co-glycolic acid)-polyethylenimine nanoparticles for prolonged nitric oxide release, antibacterial efficacy, and in vivo wound healing activity
Abstract
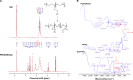
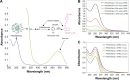
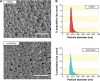
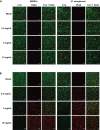
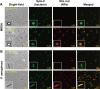
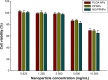
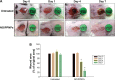
Nitric oxide (NO)-releasing nanoparticles (NPs) have emerged as a wound healing enhancer and a novel antibacterial agent that can circumvent antibiotic resistance. However, the NO release from NPs over extended periods of time is still inadequate for clinical application. In this study, we developed NO-releasing poly(lactic-co-glycolic acid)-polyethylenimine (PEI) NPs (NO/PPNPs) composed of poly(lactic-co-glycolic acid) and PEI/diazeniumdiolate (PEI/NONOate) for prolonged NO release, antibacterial efficacy, and wound healing activity. Successful preparation of PEI/NONOate was confirmed by proton nuclear magnetic resonance, Fourier transform infrared spectroscopy, and ultraviolet/visible spectrophotometry. NO/PPNPs were characterized by particle size, surface charge, and NO loading. The NO/PPNPs showed a prolonged NO release profile over 6 days without any burst release. The NO/PPNPs exhibited potent bactericidal efficacy against methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa concentration-dependently and showed the ability to bind on the surface of the bacteria. We also found that the NO released from the NO/PPNPs mediates bactericidal efficacy and is not toxic to healthy fibroblast cells. Furthermore, NO/PPNPs accelerated wound healing and epithelialization in a mouse model of a MRSA-infected wound. Therefore, our results suggest that the NO/PPNPs presented in this study could be a suitable approach for treating wounds and various skin infections.
Keywords: PEI; PLGA; antimicrobial; nitric oxide-releasing nanoparticles; wound healing.
Figures





Similar articles
-
PEI/NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant Staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix.Mater Sci Eng C Mater Biol Appl. 2019 Oct;103:109741. doi: 10.1016/j.msec.2019.109741. Epub 2019 May 14. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 31349480
-
Bacteria-Targeted Clindamycin Loaded Polymeric Nanoparticles: Effect of Surface Charge on Nanoparticle Adhesion to MRSA, Antibacterial Activity, and Wound Healing.Pharmaceutics. 2019 May 15;11(5):236. doi: 10.3390/pharmaceutics11050236. Pharmaceutics. 2019. PMID: 31096709 Free PMC article.
-
S-Nitrosoglutathione loaded poly(lactic-co-glycolic acid) microparticles for prolonged nitric oxide release and enhanced healing of methicillin-resistant Staphylococcus aureus-infected wounds.Eur J Pharm Biopharm. 2018 Nov;132:94-102. doi: 10.1016/j.ejpb.2018.09.009. Epub 2018 Sep 14. Eur J Pharm Biopharm. 2018. PMID: 30223029
-
PLGA based drug delivery systems: Promising carriers for wound healing activity.Wound Repair Regen. 2016 Mar;24(2):223-36. doi: 10.1111/wrr.12404. Wound Repair Regen. 2016. PMID: 26749322 Review.
-
Emerging Nitric Oxide and Hydrogen Sulfide Releasing Carriers for Skin Wound Healing Therapy.ChemMedChem. 2022 Jan 5;17(1):e202100429. doi: 10.1002/cmdc.202100429. Epub 2021 Nov 24. ChemMedChem. 2022. PMID: 34714595 Review.
Cited by
-
A Nitric Oxide-Responsive Transcriptional Regulator NsrR Cooperates With Lrp and CRP to Tightly Control the hmpA Gene in Vibrio vulnificus.Front Microbiol. 2021 May 21;12:681196. doi: 10.3389/fmicb.2021.681196. eCollection 2021. Front Microbiol. 2021. PMID: 34093504 Free PMC article.
-
Preparation and Safety Evaluation of Centella asiatica Total Glycosides Nitric Oxide Gel and Its Therapeutic Effect on Diabetic Cutaneous Ulcers.Evid Based Complement Alternat Med. 2022 Mar 25;2022:1419146. doi: 10.1155/2022/1419146. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35368764 Free PMC article.
-
Nitric Oxide Therapy for Diabetic Wound Healing.Adv Healthc Mater. 2019 Jun;8(12):e1801210. doi: 10.1002/adhm.201801210. Epub 2019 Jan 15. Adv Healthc Mater. 2019. PMID: 30645055 Free PMC article. Review.
-
Transcriptomic Identification and Biochemical Characterization of HmpA, a Nitric Oxide Dioxygenase, Essential for Pathogenesis of Vibrio vulnificus.Front Microbiol. 2019 Sep 24;10:2208. doi: 10.3389/fmicb.2019.02208. eCollection 2019. Front Microbiol. 2019. PMID: 31616401 Free PMC article.
-
New Weapons to Fight against Staphylococcus aureus Skin Infections.Antibiotics (Basel). 2023 Sep 22;12(10):1477. doi: 10.3390/antibiotics12101477. Antibiotics (Basel). 2023. PMID: 37887178 Free PMC article. Review.
References
-
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–1743. - PubMed
-
- Chiller K, Selkin BA, Murakawa GJ. Skin microflora and bacterial infections of the skin. J Investig Dermatol Symp Proc. 2001;6(3):170–174. - PubMed
-
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41(10):1373–1406. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

