Huntingtin Is Required for Epithelial Polarity through RAB11A-Mediated Apical Trafficking of PAR3-aPKC
- PMID: 25942483
- PMCID: PMC4420272
- DOI: 10.1371/journal.pbio.1002142
Huntingtin Is Required for Epithelial Polarity through RAB11A-Mediated Apical Trafficking of PAR3-aPKC
Abstract
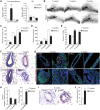
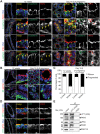
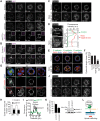
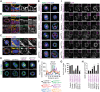
The establishment of apical-basolateral polarity is important for both normal development and disease, for example, during tumorigenesis and metastasis. During this process, polarity complexes are targeted to the apical surface by a RAB11A-dependent mechanism. Huntingtin (HTT), the protein that is mutated in Huntington disease, acts as a scaffold for molecular motors and promotes microtubule-based dynamics. Here, we investigated the role of HTT in apical polarity during the morphogenesis of the mouse mammary epithelium. We found that the depletion of HTT from luminal cells in vivo alters mouse ductal morphogenesis and lumen formation. HTT is required for the apical localization of PAR3-aPKC during epithelial morphogenesis in virgin, pregnant, and lactating mice. We show that HTT forms a complex with PAR3, aPKC, and RAB11A and ensures the microtubule-dependent apical vesicular translocation of PAR3-aPKC through RAB11A. We thus propose that HTT regulates polarized vesicular transport, lumen formation and mammary epithelial morphogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
The Par3/aPKC interaction is essential for end bud remodeling and progenitor differentiation during mammary gland morphogenesis.Genes Dev. 2009 Jun 15;23(12):1450-60. doi: 10.1101/gad.1795909. Genes Dev. 2009. PMID: 19528321 Free PMC article.
-
KIBRA suppresses apical exocytosis through inhibition of aPKC kinase activity in epithelial cells.Curr Biol. 2011 Apr 26;21(8):705-11. doi: 10.1016/j.cub.2011.03.029. Epub 2011 Apr 14. Curr Biol. 2011. PMID: 21497093
-
Hepatocyte polarity establishment and apical lumen formation are organized by Par3, Cdc42, and aPKC in conjunction with Lgl.J Biol Chem. 2021 Dec;297(6):101354. doi: 10.1016/j.jbc.2021.101354. Epub 2021 Oct 27. J Biol Chem. 2021. PMID: 34717957 Free PMC article.
-
Regulation of cell polarity during epithelial morphogenesis.Curr Opin Cell Biol. 2008 Apr;20(2):227-34. doi: 10.1016/j.ceb.2008.01.001. Epub 2008 Feb 20. Curr Opin Cell Biol. 2008. PMID: 18282696 Review.
-
The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival.Annu Rev Cell Dev Biol. 2012;28:655-85. doi: 10.1146/annurev-cellbio-092910-154033. Epub 2012 Aug 6. Annu Rev Cell Dev Biol. 2012. PMID: 22881460 Review.
Cited by
-
Nuclear fallout provides a new link between aPKC and polarized cell trafficking.BMC Biol. 2016 Apr 18;14:32. doi: 10.1186/s12915-016-0253-6. BMC Biol. 2016. PMID: 27089924 Free PMC article.
-
Is Huntingtin Dispensable in the Adult Brain?J Huntingtons Dis. 2017;6(1):1-17. doi: 10.3233/JHD-170235. J Huntingtons Dis. 2017. PMID: 28339401 Free PMC article. Review.
-
Huntingtin CAG expansion impairs germ layer patterning in synthetic human 2D gastruloids through polarity defects.Development. 2021 Oct 1;148(19):dev199513. doi: 10.1242/dev.199513. Epub 2021 Oct 5. Development. 2021. PMID: 34608934 Free PMC article.
-
The transcriptional repressor Blimp1 is expressed in rare luminal progenitors and is essential for mammary gland development.Development. 2016 May 15;143(10):1663-73. doi: 10.1242/dev.136358. Development. 2016. PMID: 27190036 Free PMC article.
-
Huntington's disease alters human neurodevelopment.Science. 2020 Aug 14;369(6505):787-793. doi: 10.1126/science.aax3338. Epub 2020 Jul 16. Science. 2020. PMID: 32675289 Free PMC article.
References
-
- Bilder D PDZ domain polarity complexes. Curr Biol. 2003;13: R661–662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

