Recurrent turnover of senescent cells during regeneration of a complex structure
- PMID: 25942455
- PMCID: PMC4434796
- DOI: 10.7554/eLife.05505
Recurrent turnover of senescent cells during regeneration of a complex structure
Abstract
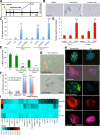
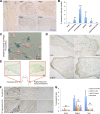
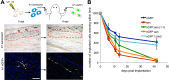
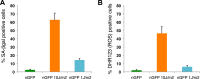
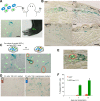
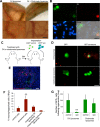
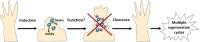
Cellular senescence has been recently linked to the promotion of age-related pathologies, including a decline in regenerative capacity. While such capacity deteriorates with age in mammals, it remains intact in species such as salamanders, which have an extensive repertoire of regeneration and can undergo multiple episodes through their lifespan. Here we show that, surprisingly, there is a significant induction of cellular senescence during salamander limb regeneration, but that rapid and effective mechanisms of senescent cell clearance operate in normal and regenerating tissues. Furthermore, the number of senescent cells does not increase upon repetitive amputation or ageing, in contrast to mammals. Finally, we identify the macrophage as a critical player in this efficient senescent cell clearance mechanism. We propose that effective immunosurveillance of senescent cells in salamanders supports their ability to undergo regeneration throughout their lifespan.
Keywords: ageing; axolotl; cell biology; developmental biology; macrophage; newt; salamander; senescence; stem cells.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures




Similar articles
-
Induction and Characterization of Cellular Senescence in Salamanders.Methods Mol Biol. 2023;2562:135-154. doi: 10.1007/978-1-0716-2659-7_8. Methods Mol Biol. 2023. PMID: 36272072
-
A comparative study of gland cells implicated in the nerve dependence of salamander limb regeneration.J Anat. 2010 Jul;217(1):16-25. doi: 10.1111/j.1469-7580.2010.01239.x. Epub 2010 Apr 26. J Anat. 2010. PMID: 20456522 Free PMC article.
-
Macrophages are required for adult salamander limb regeneration.Proc Natl Acad Sci U S A. 2013 Jun 4;110(23):9415-20. doi: 10.1073/pnas.1300290110. Epub 2013 May 20. Proc Natl Acad Sci U S A. 2013. PMID: 23690624 Free PMC article.
-
Putative epithelial-mesenchymal transitions during salamander limb regeneration: Current perspectives and future investigations.Ann N Y Acad Sci. 2024 Oct;1540(1):89-103. doi: 10.1111/nyas.15210. Epub 2024 Sep 13. Ann N Y Acad Sci. 2024. PMID: 39269330 Review.
-
Immunity in salamander regeneration: Where are we standing and where are we headed?Dev Dyn. 2021 Jun;250(6):753-767. doi: 10.1002/dvdy.251. Epub 2020 Sep 21. Dev Dyn. 2021. PMID: 32924213 Free PMC article. Review.
Cited by
-
Senescent cells suppress macrophage-mediated corpse removal via upregulation of the CD47-QPCT/L axis.J Cell Biol. 2023 Feb 6;222(2):e202207097. doi: 10.1083/jcb.202207097. Epub 2022 Dec 2. J Cell Biol. 2023. PMID: 36459066 Free PMC article.
-
Hallmarks of regeneration.Cell Stem Cell. 2024 Sep 5;31(9):1244-1261. doi: 10.1016/j.stem.2024.07.007. Epub 2024 Aug 19. Cell Stem Cell. 2024. PMID: 39163854 Review.
-
Could senescence phenotypes strike the balance to promote tumor dormancy?Cancer Metastasis Rev. 2023 Mar;42(1):143-160. doi: 10.1007/s10555-023-10089-z. Epub 2023 Feb 3. Cancer Metastasis Rev. 2023. PMID: 36735097 Free PMC article. Review.
-
Connective tissue fibroblasts from highly regenerative mammals are refractory to ROS-induced cellular senescence.Nat Commun. 2019 Sep 27;10(1):4400. doi: 10.1038/s41467-019-12398-w. Nat Commun. 2019. PMID: 31562333 Free PMC article.
-
Senescent cells and macrophages: key players for regeneration?Open Biol. 2020 Dec;10(12):200309. doi: 10.1098/rsob.200309. Epub 2020 Dec 23. Open Biol. 2020. PMID: 33352064 Free PMC article. Review.
References
-
- Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biology. 2013;15:978–990. doi: 10.1038/ncb2784. - DOI - PMC - PubMed
-
- Baker DJ, Perez-Terzic C, Jin F, Pitel KS, Niederlander NJ, Jeganathan K, Yamada S, Reyes S, Rowe L, Hiddinga HJ, Eberhardt NL, Terzic A, van Deursen JM. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nature Cell Biology. 2008;10:825–836. doi: 10.1038/ncb1744. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

