Systemic chemotherapy is modulated by platelet-activating factor-receptor agonists
- PMID: 25922565
- PMCID: PMC4398925
- DOI: 10.1155/2015/820543
Systemic chemotherapy is modulated by platelet-activating factor-receptor agonists
Abstract
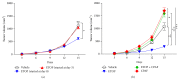
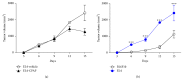
Chemotherapy is used to treat numerous cancers including melanoma. However, its effectiveness in clinical settings is often hampered by various mechanisms. Previous studies have demonstrated that prooxidative stressor-mediated generation of oxidized lipids with platelet-activating factor-receptor (PAF-R) agonistic activity induces systemic immunosuppression that augments the growth of experimental melanoma tumors. We have recently shown that treatment of murine B16F10 melanoma cells in vitro or tumors implanted into syngeneic mice and treated intratumorally with various chemotherapeutic agents generated PAF-R agonists in a process blocked by antioxidants. Notably, these intratumoral chemotherapy-generated PAF-R agonists augmented the growth of secondary (untreated) tumors in a PAF-R dependent manner. As both localized and systemic chemotherapies are used based on tumor localization/stage and metastases, the current studies were sought to determine effects of PAF-R agonists on systemic chemotherapy against experimental melanoma. Here, we show that systemic chemotherapy with etoposide (ETOP) attenuates the growth of melanoma tumors when given subsequent to the tumor cell implantation. Importantly, this ETOP-mediated suppression of melanoma tumor growth was blocked by exogenous administration of a PAF-R agonist, CPAF. These findings indicate that PAF-R agonists not only negatively affect the ability of localized chemotherapy but also compromise the efficacy of systemic chemotherapy against murine melanoma.
Figures
Similar articles
-
Radiation therapy generates platelet-activating factor agonists.Oncotarget. 2016 Apr 12;7(15):20788-800. doi: 10.18632/oncotarget.7878. Oncotarget. 2016. PMID: 26959112 Free PMC article.
-
Cigarette smoke exposure inhibits contact hypersensitivity via the generation of platelet-activating factor agonists.J Immunol. 2013 Mar 1;190(5):2447-54. doi: 10.4049/jimmunol.1202699. Epub 2013 Jan 25. J Immunol. 2013. PMID: 23355733 Free PMC article.
-
Expression of the platelet-activating factor receptor enhances benzyl isothiocyanate-induced apoptosis in murine and human melanoma cells.Mol Med Rep. 2015 Jul;12(1):394-400. doi: 10.3892/mmr.2015.3371. Epub 2015 Feb 18. Mol Med Rep. 2015. PMID: 25695262 Free PMC article.
-
Inflammation and melanoma growth and metastasis: the role of platelet-activating factor (PAF) and its receptor.Cancer Metastasis Rev. 2007 Dec;26(3-4):359-71. doi: 10.1007/s10555-007-9092-9. Cancer Metastasis Rev. 2007. PMID: 17721743 Review.
-
Specific receptors of platelet-activating factor, receptor heterogeneity, and signal transduction mechanisms.J Lipid Mediat. 1990 May-Jul;2(3-4):123-58. J Lipid Mediat. 1990. PMID: 1966805 Review.
Cited by
-
Advances in Immunotherapy for Melanoma: A Comprehensive Review.Mediators Inflamm. 2017;2017:3264217. doi: 10.1155/2017/3264217. Epub 2017 Aug 1. Mediators Inflamm. 2017. PMID: 28848246 Free PMC article. Review.
-
Platelet-Activating Factor Receptor Ligands Protect Tumor Cells from Radiation-Induced Cell Death.Front Oncol. 2018 Feb 5;8:10. doi: 10.3389/fonc.2018.00010. eCollection 2018. Front Oncol. 2018. PMID: 29459885 Free PMC article.
-
Radiation therapy generates platelet-activating factor agonists.Oncotarget. 2016 Apr 12;7(15):20788-800. doi: 10.18632/oncotarget.7878. Oncotarget. 2016. PMID: 26959112 Free PMC article.
-
Cisplatin-induced oxPAPC release enhances MDSCs infiltration into LL2 tumour tissues through MCP-1/CCL2 and LTB4/LTB4R pathways.Cell Prolif. 2024 Apr;57(4):e13570. doi: 10.1111/cpr.13570. Epub 2023 Oct 31. Cell Prolif. 2024. PMID: 37905494 Free PMC article.
-
MicroRNA-Directed Cancer Therapies: Implications in Melanoma Intervention.J Pharmacol Exp Ther. 2018 Jan;364(1):1-12. doi: 10.1124/jpet.117.242636. Epub 2017 Oct 20. J Pharmacol Exp Ther. 2018. PMID: 29054858 Free PMC article. Review.
References
-
- Howlader N., Noone A. M., Krapcho M., et al. SEER Cancer Statistics Review, 1975–2010. Bethesda, Md, USA: National Cancer Institute; 2012. http://seer.cancer.gov/csr/1975_2010/, http://seer.cancer.gov/statfacts/html/melan.html.
-
- Hwu W. J. New approaches in the treatment of metastatic melanoma: thalidomide and temozolomide. Oncology. 2000;12(supplement 13):25–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

