Mechanistic Assessment of PD-1H Coinhibitory Receptor-Induced T Cell Tolerance to Allogeneic Antigens
- PMID: 25917101
- PMCID: PMC4433880
- DOI: 10.4049/jimmunol.1402648
Mechanistic Assessment of PD-1H Coinhibitory Receptor-Induced T Cell Tolerance to Allogeneic Antigens
Abstract
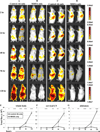
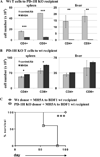
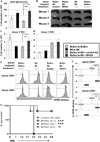
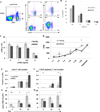
PD-1H is a recently identified cell surface coinhibitory molecule of the B7/CD28 immune modulatory gene family. We showed previously that single injection of a PD-1H agonistic mAb protected mice from graft-versus-host disease (GVHD). In this study, we report two distinct mechanisms operate in PD-1H-induced T cell tolerance. First, signaling via PD-1H coinhibitory receptor potently arrests alloreactive donor T cells from activation and expansion in the initiation phase. Second, donor regulatory T cells are subsequently expanded to maintain long-term tolerance and GVHD suppression. Our study reveals the crucial function of PD-1H as a coinhibitory receptor on alloreactive T cells and its function in the regulation of T cell tolerance. Therefore, PD-1H may be a target for the modulation of alloreactive T cells in GVHD and transplantation.
Copyright © 2015 by The American Association of Immunologists, Inc.
Conflict of interest statement
Figures



Similar articles
-
Involvement of the programmed death-1/programmed death-1 ligand pathway in CD4+CD25+ regulatory T-cell activity to suppress alloimmune responses.Transplantation. 2007 Mar 27;83(6):774-82. doi: 10.1097/01.tp.0000256293.90270.e8. Transplantation. 2007. PMID: 17414712
-
B7H1/CD80 interaction augments PD-1-dependent T cell apoptosis and ameliorates graft-versus-host disease.J Immunol. 2015 Jan 15;194(2):560-74. doi: 10.4049/jimmunol.1402157. Epub 2014 Dec 8. J Immunol. 2015. PMID: 25488990 Free PMC article.
-
Comparative analysis of dendritic cells and anti-CD3/CD28 expanded regulatory T cells for application in transplantation.Transpl Immunol. 2009 Dec;22(1-2):82-92. doi: 10.1016/j.trim.2009.07.004. Epub 2009 Jul 25. Transpl Immunol. 2009. PMID: 19635560
-
Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways.Immunol Rev. 2008 Aug;224:166-82. doi: 10.1111/j.1600-065X.2008.00662.x. Immunol Rev. 2008. PMID: 18759926 Review.
-
Role of the PD-1 pathway in the immune response.Am J Transplant. 2012 Oct;12(10):2575-87. doi: 10.1111/j.1600-6143.2012.04224.x. Epub 2012 Aug 17. Am J Transplant. 2012. PMID: 22900886 Free PMC article. Review.
Cited by
-
Agonistic nanobodies and antibodies to human VISTA.MAbs. 2021 Jan-Dec;13(1):2003281. doi: 10.1080/19420862.2021.2003281. MAbs. 2021. PMID: 34818120 Free PMC article.
-
A crucial role of the PD-1H coinhibitory receptor in suppressing experimental asthma.Cell Mol Immunol. 2018 Sep;15(9):838-845. doi: 10.1038/cmi.2017.16. Epub 2017 May 8. Cell Mol Immunol. 2018. PMID: 28479600 Free PMC article.
-
Emerging strategies for treating autoimmune disease with genetically modified dendritic cells.Cell Commun Signal. 2024 May 7;22(1):262. doi: 10.1186/s12964-024-01641-7. Cell Commun Signal. 2024. PMID: 38715122 Free PMC article. Review.
-
Clinical and research updates on the VISTA immune checkpoint: immuno-oncology themes and highlights.Front Oncol. 2023 Sep 15;13:1225081. doi: 10.3389/fonc.2023.1225081. eCollection 2023. Front Oncol. 2023. PMID: 37795437 Free PMC article. Review.
-
VISTA: A Promising Target for Cancer Immunotherapy?Immunotargets Ther. 2021 Jun 22;10:185-200. doi: 10.2147/ITT.S260429. eCollection 2021. Immunotargets Ther. 2021. PMID: 34189130 Free PMC article. Review.
References
-
- Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, Nishimura H, Taylor PA. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J. Immunol. 2003;171:1272–1277. - PubMed
-
- Blazar BR, Taylor PA, Linsley PS, Vallera DA. In vivo blockade of CD28/CTLA4: B7/BB1 interaction with CTLA4-Ig reduces lethal murine graft-versus-host disease across the major histocompatibility complex barrier in mice. Blood. 1994;83:3815–3825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

