A primer to single-particle cryo-electron microscopy
- PMID: 25910204
- PMCID: PMC4409659
- DOI: 10.1016/j.cell.2015.03.050
A primer to single-particle cryo-electron microscopy
Abstract
Cryo-electron microscopy (cryo-EM) of single-particle specimens is used to determine the structure of proteins and macromolecular complexes without the need for crystals. Recent advances in detector technology and software algorithms now allow images of unprecedented quality to be recorded and structures to be determined at near-atomic resolution. However, compared with X-ray crystallography, cryo-EM is a young technique with distinct challenges. This primer explains the different steps and considerations involved in structure determination by single-particle cryo-EM to provide an overview for scientists wishing to understand more about this technique and the interpretation of data obtained with it, as well as a starting guide for new practitioners.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
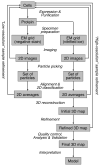
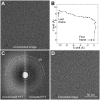


Similar articles
-
Cryo electron microscopy to determine the structure of macromolecular complexes.Methods. 2016 Feb 15;95:78-85. doi: 10.1016/j.ymeth.2015.11.023. Epub 2015 Nov 27. Methods. 2016. PMID: 26638773 Free PMC article. Review.
-
Electron cryo-microscopy for elucidating the dynamic nature of live-protein complexes.Biochim Biophys Acta Gen Subj. 2020 Feb;1864(2):129436. doi: 10.1016/j.bbagen.2019.129436. Epub 2019 Sep 13. Biochim Biophys Acta Gen Subj. 2020. PMID: 31525409 Review.
-
Sample preparation of biological macromolecular assemblies for the determination of high-resolution structures by cryo-electron microscopy.Microscopy (Oxf). 2016 Feb;65(1):23-34. doi: 10.1093/jmicro/dfv367. Epub 2015 Dec 15. Microscopy (Oxf). 2016. PMID: 26671943 Review.
-
Cryo-electron microscopy and X-ray crystallography: complementary approaches to structural biology and drug discovery.Acta Crystallogr F Struct Biol Commun. 2017 Apr 1;73(Pt 4):174-183. doi: 10.1107/S2053230X17003740. Epub 2017 Mar 29. Acta Crystallogr F Struct Biol Commun. 2017. PMID: 28368275 Free PMC article. Review.
-
Cryo-electron microscopy and the amazing race to atomic resolution.Biochemistry. 2015 May 26;54(20):3133-41. doi: 10.1021/acs.biochem.5b00114. Epub 2015 May 14. Biochemistry. 2015. PMID: 25955078 Review.
Cited by
-
Dissecting Virus Infectious Cycles by Cryo-Electron Microscopy.PLoS Pathog. 2016 Jun 30;12(6):e1005625. doi: 10.1371/journal.ppat.1005625. eCollection 2016 Jun. PLoS Pathog. 2016. PMID: 27362353 Free PMC article. Review. No abstract available.
-
Experimental evaluation of super-resolution imaging and magnification choice in single-particle cryo-EM.J Struct Biol X. 2021 Mar 13;5:100047. doi: 10.1016/j.yjsbx.2021.100047. eCollection 2021. J Struct Biol X. 2021. PMID: 33817625 Free PMC article.
-
An electron counting algorithm improves imaging of proteins with low-acceleration-voltage cryo-electron microscope.Commun Biol. 2022 Apr 6;5(1):321. doi: 10.1038/s42003-022-03284-1. Commun Biol. 2022. PMID: 35388174 Free PMC article.
-
Learning to automate cryo-electron microscopy data collection with Ptolemy.IUCrJ. 2023 Jan 1;10(Pt 1):90-102. doi: 10.1107/S2052252522010612. IUCrJ. 2023. PMID: 36598505 Free PMC article.
-
Recent Technical Advances in Sample Preparation for Single-Particle Cryo-EM.Front Mol Biosci. 2022 Jun 24;9:892459. doi: 10.3389/fmolb.2022.892459. eCollection 2022. Front Mol Biosci. 2022. PMID: 35813814 Free PMC article. Review.
References
-
- Adiga PS, Malladi R, Baxter W, Glaeser RM. A binary segmentation approach for boxing ribosome particles in cryo EM micrographs. J Struct Biol. 2004;145:142–151. - PubMed
-
- Bai XC, McMullan G, Scheres SH. How cryo-EM is revolutionizing structural biology. Trends Biochem Sci. 2015;40:49–57. - PubMed
-
- Beckmann R, Bubeck D, Grassucci R, Penczek PA, Verschoor A, Blobel G, Frank J. Alignment of conduits for the nascent polypeptide chain in the ribosome-Sec61 complex. Science. 1997;278:2123–2126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

