The neuropathology of chronic traumatic encephalopathy
- PMID: 25904048
- PMCID: PMC4526170
- DOI: 10.1111/bpa.12248
The neuropathology of chronic traumatic encephalopathy
Abstract
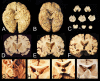
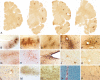
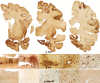
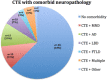
Repetitive brain trauma is associated with a progressive neurological deterioration, now termed as chronic traumatic encephalopathy (CTE). Most instances of CTE occur in association with the play of sports, but CTE has also been reported in association with blast injuries and other neurotrauma. Symptoms of CTE include behavioral and mood changes, memory loss, cognitive impairment and dementia. Like many other neurodegenerative diseases, CTE is diagnosed with certainty only by neuropathological examination of brain tissue. CTE is a tauopathy characterized by the deposition of hyperphosphorylated tau (p-tau) protein as neurofibrillary tangles, astrocytic tangles and neurites in striking clusters around small blood vessels of the cortex, typically at the sulcal depths. Severely affected cases show p-tau pathology throughout the brain. Abnormalities in phosphorylated 43 kDa TAR DNA-binding protein are found in most cases of CTE; beta-amyloid is identified in 43%, associated with age. Given the importance of sports participation and physical exercise to physical and psychological health as well as disease resilience, it is critical to identify the genetic risk factors for CTE as well as to understand how other variables, such as stress, age at exposure, gender, substance abuse and other exposures, contribute to the development of CTE.
Keywords: Alzheimer's disease; TDP-43; chronic traumatic encephalopathy; tauopathy; traumatic brain injury.
© 2015 International Society of Neuropathology.
Figures



Similar articles
-
The Neuropathology of Chronic Traumatic Encephalopathy: The Status of the Literature.Semin Neurol. 2020 Aug;40(4):359-369. doi: 10.1055/s-0040-1713632. Epub 2020 Jul 26. Semin Neurol. 2020. PMID: 32712946 Review.
-
The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy.Acta Neuropathol. 2016 Jan;131(1):75-86. doi: 10.1007/s00401-015-1515-z. Epub 2015 Dec 14. Acta Neuropathol. 2016. PMID: 26667418 Free PMC article.
-
Post-traumatic neurodegeneration and chronic traumatic encephalopathy.Mol Cell Neurosci. 2015 May;66(Pt B):81-90. doi: 10.1016/j.mcn.2015.03.007. Epub 2015 Mar 7. Mol Cell Neurosci. 2015. PMID: 25758552 Review.
-
Tau immunophenotypes in chronic traumatic encephalopathy recapitulate those of ageing and Alzheimer's disease.Brain. 2020 May 1;143(5):1572-1587. doi: 10.1093/brain/awaa071. Brain. 2020. PMID: 32390044 Free PMC article.
-
The neuropathology of chronic traumatic encephalopathy.Handb Clin Neurol. 2018;158:297-307. doi: 10.1016/B978-0-444-63954-7.00028-8. Handb Clin Neurol. 2018. PMID: 30482357 Review.
Cited by
-
Localization of the hydrogen sulfide and oxytocin systems at the depth of the sulci in a porcine model of acute subdural hematoma.Neural Regen Res. 2021 Dec;16(12):2376-2382. doi: 10.4103/1673-5374.313018. Neural Regen Res. 2021. PMID: 33907009 Free PMC article. Review.
-
Single-domain antibodies and aptamers drive new opportunities for neurodegenerative disease research.Front Immunol. 2024 Aug 22;15:1426656. doi: 10.3389/fimmu.2024.1426656. eCollection 2024. Front Immunol. 2024. PMID: 39238639 Free PMC article. Review.
-
Functional, Structural, and Neurotoxicity Biomarkers in Integrative Assessment of Concussions.Front Neurol. 2016 Oct 5;7:172. doi: 10.3389/fneur.2016.00172. eCollection 2016. Front Neurol. 2016. PMID: 27761129 Free PMC article.
-
Simulation of the Strain Amplification in Sulci Due to Blunt Impact to the Head.Front Neurol. 2020 Sep 8;11:998. doi: 10.3389/fneur.2020.00998. eCollection 2020. Front Neurol. 2020. PMID: 33013659 Free PMC article.
-
NK1 antagonists attenuate tau phosphorylation after blast and repeated concussive injury.Sci Rep. 2021 Apr 23;11(1):8861. doi: 10.1038/s41598-021-88237-0. Sci Rep. 2021. PMID: 33893374 Free PMC article.
References
-
- Anthony IC, Norrby KE, Dingwall T, Carnie FW, Millar T, Arango JC et al (2010) Predisposition to accelerated Alzheimer‐related changes in the brains of human immunodeficiency virus negative opiate abusers. Brain 133(Pt 12):3685–3698. - PubMed
-
- Bieniek KF, Stein TD, Alvarez VE, Fry BT, Dickson DW, McKee AC (2014) Poster abstracts. Brain Pathol 24:39–103.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

