NK cells require IL-28R for optimal in vivo activity
- PMID: 25901316
- PMCID: PMC4426428
- DOI: 10.1073/pnas.1424241112
NK cells require IL-28R for optimal in vivo activity
Expression of concern in
-
Editorial Expression of Concern: NK cells require IL-28R for optimal in vivo activity.Proc Natl Acad Sci U S A. 2024 Nov 12;121(46):e2420879121. doi: 10.1073/pnas.2420879121. Epub 2024 Nov 7. Proc Natl Acad Sci U S A. 2024. PMID: 39508771 Free PMC article. No abstract available.
Abstract
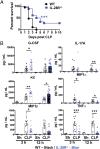
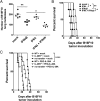
Natural killer (NK) cells are naturally circulating innate lymphoid cells that protect against tumor initiation and metastasis and contribute to immunopathology during inflammation. The signals that prime NK cells are not completely understood, and, although the importance of IFN type I is well recognized, the role of type III IFN is comparatively very poorly studied. IL-28R-deficient mice were resistant to LPS and cecal ligation puncture-induced septic shock, and hallmark cytokines in these disease models were dysregulated in the absence of IL-28R. IL-28R-deficient mice were more sensitive to experimental tumor metastasis and carcinogen-induced tumor formation than WT mice, and additional blockade of interferon alpha/beta receptor 1 (IFNAR1), but not IFN-γ, further enhanced metastasis and tumor development. IL-28R-deficient mice were also more susceptible to growth of the NK cell-sensitive lymphoma, RMAs. Specific loss of IL-28R in NK cells transferred into lymphocyte-deficient mice resulted in reduced LPS-induced IFN-γ levels and enhanced tumor metastasis. Therefore, by using IL-28R-deficient mice, which are unable to signal type III IFN-λ, we demonstrate for the first time, to our knowledge, the ability of IFN-λ to directly regulate NK cell effector functions in vivo, alone and in the context of IFN-αβ.
Keywords: IL-28R; LPS; NK cells; anti-tumor; interferon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Neutralization of IL-10 restores the downregulation of IL-18 receptor on natural killer cells and interferon-γ production in septic mice, thus leading to an improved survival.Shock. 2012 Feb;37(2):177-82. doi: 10.1097/SHK.0b013e31823f18ad. Shock. 2012. PMID: 22089189
-
Tumor-induced suppression of interferon-gamma production and enhancement of interleukin-10 production by natural killer (NK) cells: paralleled to CD4+ T cells.Mol Immunol. 2005 May;42(9):1023-31. doi: 10.1016/j.molimm.2004.09.035. Epub 2004 Nov 23. Mol Immunol. 2005. PMID: 15829292
-
CD137-deficient mice have reduced NK/NKT cell numbers and function, are resistant to lipopolysaccharide-induced shock syndromes, and have lower IL-4 responses.J Immunol. 2004 Sep 15;173(6):4218-29. doi: 10.4049/jimmunol.173.6.4218. J Immunol. 2004. PMID: 15356173
-
IFIT2 is an effector protein of type I IFN-mediated amplification of lipopolysaccharide (LPS)-induced TNF-α secretion and LPS-induced endotoxin shock.J Immunol. 2013 Oct 1;191(7):3913-21. doi: 10.4049/jimmunol.1203305. Epub 2013 Sep 6. J Immunol. 2013. PMID: 24014876
-
Implications of a 'Third Signal' in NK Cells.Cells. 2021 Jul 31;10(8):1955. doi: 10.3390/cells10081955. Cells. 2021. PMID: 34440725 Free PMC article. Review.
Cited by
-
Portraying the dark side of endogenous IFN-λ for promoting cancer progression and immunoevasion in pan-cancer.J Transl Med. 2023 Sep 11;21(1):615. doi: 10.1186/s12967-023-04453-4. J Transl Med. 2023. PMID: 37697300 Free PMC article. Review.
-
Type I and III Interferon in the Gut: Tight Balance between Host Protection and Immunopathology.Front Immunol. 2017 Mar 14;8:258. doi: 10.3389/fimmu.2017.00258. eCollection 2017. Front Immunol. 2017. PMID: 28352268 Free PMC article. Review.
-
The NK cell-cancer cycle: advances and new challenges in NK cell-based immunotherapies.Nat Immunol. 2020 Aug;21(8):835-847. doi: 10.1038/s41590-020-0728-z. Epub 2020 Jul 20. Nat Immunol. 2020. PMID: 32690952 Free PMC article. Review.
-
IFN-λ3 polymorphism indirectly influences NK cell phenotype and function during acute HCV infection.Immun Inflamm Dis. 2016 Aug 16;4(3):376-88. doi: 10.1002/iid3.122. eCollection 2016 Sep. Immun Inflamm Dis. 2016. PMID: 27621819 Free PMC article.
-
Antiviral Responses in Cancer: Boosting Antitumor Immunity Through Activation of Interferon Pathway in the Tumor Microenvironment.Front Immunol. 2021 Dec 2;12:782852. doi: 10.3389/fimmu.2021.782852. eCollection 2021. Front Immunol. 2021. PMID: 34925363 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

