Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer
- PMID: 25897158
- PMCID: PMC4672027
- DOI: 10.1200/JCO.2014.58.3708
Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer
Abstract
Purpose: Programmed death 1 is an immune checkpoint that suppresses antitumor immunity. Nivolumab, a fully human immunoglobulin G4 programmed death 1 immune checkpoint inhibitor antibody, was active and generally well tolerated in patients with advanced solid tumors treated in a phase I trial with expansion cohorts. We report overall survival (OS), response durability, and long-term safety in patients with non-small-cell lung cancer (NSCLC) receiving nivolumab in this trial.
Patients and methods: Patients (N = 129) with heavily pretreated advanced NSCLC received nivolumab 1, 3, or 10 mg/kg intravenously once every 2 weeks in 8-week cycles for up to 96 weeks. Tumor burden was assessed by RECIST (version 1.0) after each cycle.
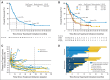
Results: Median OS across doses was 9.9 months; 1-, 2-, and 3-year OS rates were 42%, 24%, and 18%, respectively, across doses and 56%, 42%, and 27%, respectively, at the 3-mg/kg dose (n = 37) chosen for further clinical development. Among 22 patients (17%) with objective responses, estimated median response duration was 17.0 months. An additional six patients (5%) had unconventional immune-pattern responses. Response rates were similar in squamous and nonsquamous NSCLC. Eighteen responding patients discontinued nivolumab for reasons other than progressive disease; nine (50%) of those had responses lasting > 9 months after their last dose. Grade 3 to 4 treatment-related adverse events occurred in 14% of patients. Three treatment-related deaths (2% of patients) occurred, each associated with pneumonitis.
Conclusion: Nivolumab monotherapy produced durable responses and encouraging survival rates in patients with heavily pretreated NSCLC. Randomized clinical trials with nivolumab in advanced NSCLC are ongoing.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures
Comment in
-
Durable Responses With PD-1 Inhibition in Lung and Kidney Cancer and the Ongoing Search for Predictive Biomarkers.J Clin Oncol. 2015 Jun 20;33(18):1993-4. doi: 10.1200/JCO.2015.61.4172. Epub 2015 Apr 27. J Clin Oncol. 2015. PMID: 25918290 No abstract available.
Similar articles
-
Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer.J Clin Oncol. 2016 Sep 1;34(25):2969-79. doi: 10.1200/JCO.2016.66.9861. Epub 2016 Jun 27. J Clin Oncol. 2016. PMID: 27354481 Free PMC article. Clinical Trial.
-
Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer.J Clin Oncol. 2016 Sep 1;34(25):2980-7. doi: 10.1200/JCO.2016.66.9929. Epub 2016 Jun 27. J Clin Oncol. 2016. PMID: 27354485 Free PMC article. Clinical Trial.
-
Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial.Lancet Oncol. 2015 Mar;16(3):257-65. doi: 10.1016/S1470-2045(15)70054-9. Epub 2015 Feb 20. Lancet Oncol. 2015. PMID: 25704439 Free PMC article. Clinical Trial.
-
Nivolumab: a review in advanced squamous non-small cell lung cancer.Drugs. 2015 Nov;75(16):1925-34. doi: 10.1007/s40265-015-0492-9. Drugs. 2015. PMID: 26514815 Review.
-
Nivolumab in the treatment of metastatic squamous non-small cell lung cancer: a review of the evidence.Ther Adv Respir Dis. 2016 Oct;10(5):444-54. doi: 10.1177/1753465816661091. Epub 2016 Jul 31. Ther Adv Respir Dis. 2016. PMID: 27480166 Free PMC article. Review.
Cited by
-
Nephrotoxicity as a Complication of Chemotherapy and Immunotherapy in the Treatment of Colorectal Cancer, Melanoma and Non-Small Cell Lung Cancer.Int J Mol Sci. 2021 Apr 28;22(9):4618. doi: 10.3390/ijms22094618. Int J Mol Sci. 2021. PMID: 33924827 Free PMC article. Review.
-
Catching up with solid tumor oncology: what is the evidence for a prognostic role of programmed cell death-ligand 1/programmed cell death-1 expression in B-cell lymphomas?Haematologica. 2016 Oct;101(10):1144-1158. doi: 10.3324/haematol.2016.145904. Haematologica. 2016. PMID: 27694502 Free PMC article. Review.
-
Steven-Johnson Syndrome: A Rare but Serious Adverse Event of Nivolumab Use in a Patient With Metastatic Gastric Adenocarcinoma.J Med Cases. 2022 Sep;13(9):449-455. doi: 10.14740/jmc3992. Epub 2022 Sep 28. J Med Cases. 2022. PMID: 36258702 Free PMC article.
-
Systematic construction and validation of an immune prognostic model for lung adenocarcinoma.J Cell Mol Med. 2020 Jan;24(2):1233-1244. doi: 10.1111/jcmm.14719. Epub 2019 Nov 28. J Cell Mol Med. 2020. PMID: 31779055 Free PMC article.
-
The safety of first and subsequent lines of PD-1/PD-L1 inhibitors monotherapy in non-small cell lung cancer patients: a meta-analysis.Transl Cancer Res. 2020 May;9(5):3231-3241. doi: 10.21037/tcr.2020.03.82. Transl Cancer Res. 2020. PMID: 35117689 Free PMC article.
References
-
- Gettinger S, Lynch T. A decade of advances in treatment for advanced non-small cell lung cancer. Clin Chest Med. 2011;32:839–851. - PubMed
-
- Yang JC, Hirsh V, Schuler M, et al. Symptom control and quality of life in LUX-Lung 3: A phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3342–3350. - PubMed
-
- Soria JC, Mauguen A, Reck M, et al. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24:20–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

