Mesenchymal stem cells mediate the clinical phenotype of inflammatory breast cancer in a preclinical model
- PMID: 25887413
- PMCID: PMC4389342
- DOI: 10.1186/s13058-015-0549-4
Mesenchymal stem cells mediate the clinical phenotype of inflammatory breast cancer in a preclinical model
Abstract
Introduction: Inflammatory breast cancer (IBC) is an aggressive type of breast cancer, characterized by very rapid progression, enlargement of the breast, skin edema causing an orange peel appearance (peau d'orange), erythema, thickening, and dermal lymphatic invasion. It is characterized by E-cadherin overexpression in the primary and metastatic disease, but to date no robust molecular features that specifically identify IBC have been reported. Further, models that recapitulate all of these clinical findings are limited and as a result no studies have demonstrated modulation of these clinical features as opposed to simply tumor cell growth.
Methods: Hypothesizing the clinical presentation of IBC may be mediated in part by the microenvironment, we examined the effect of co-injection of IBC xenografts with mesenchymal stem/stromal cells (MSCs).
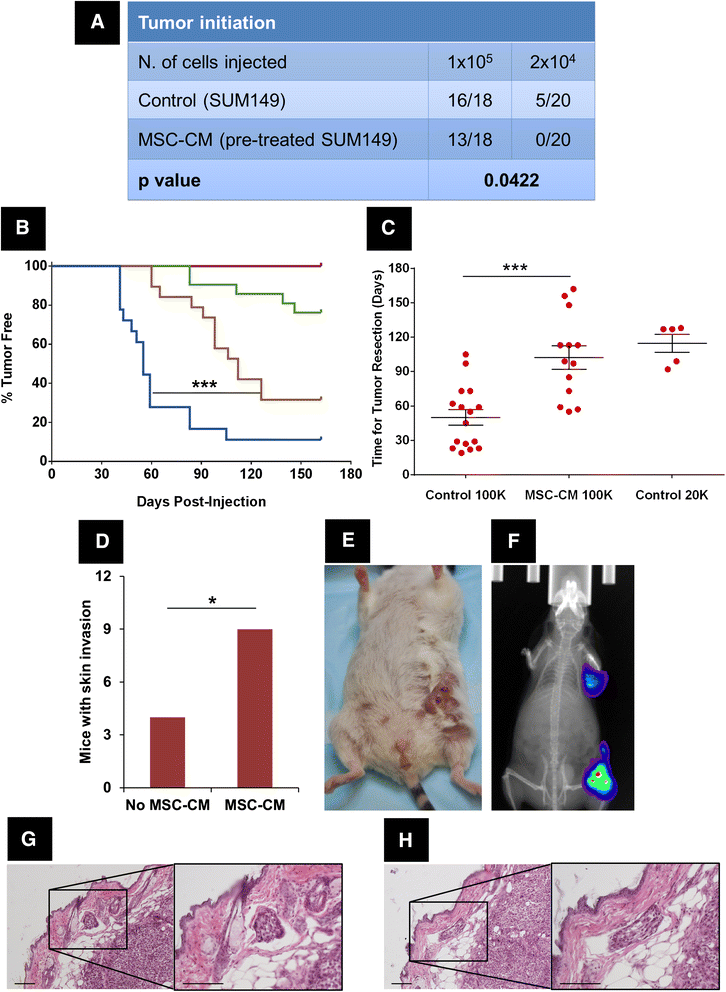
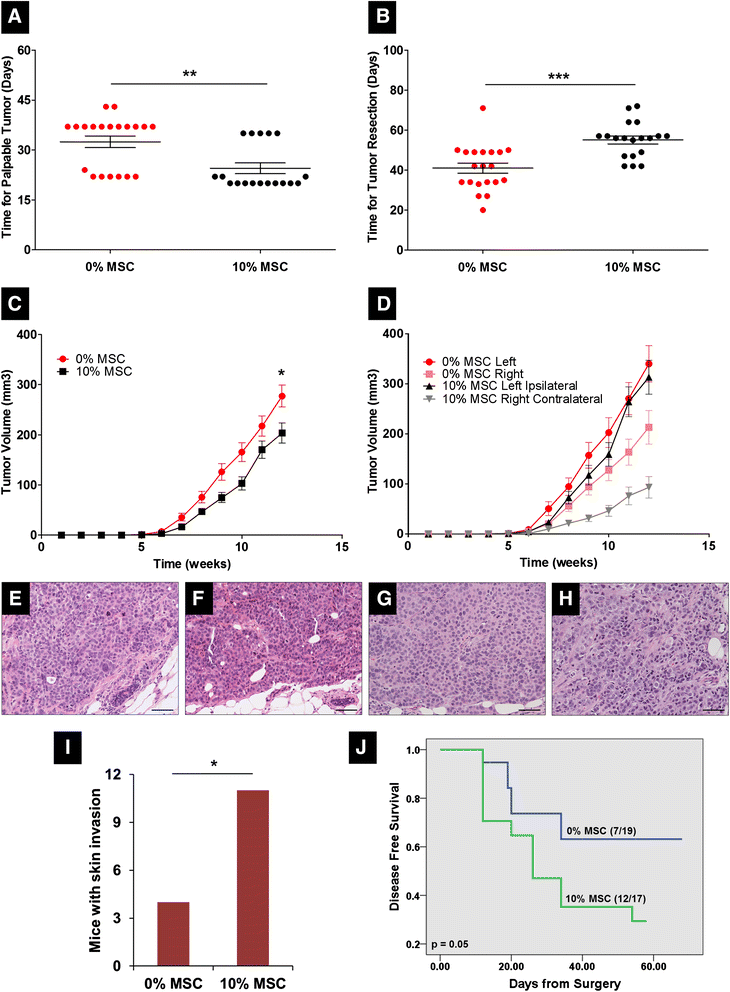
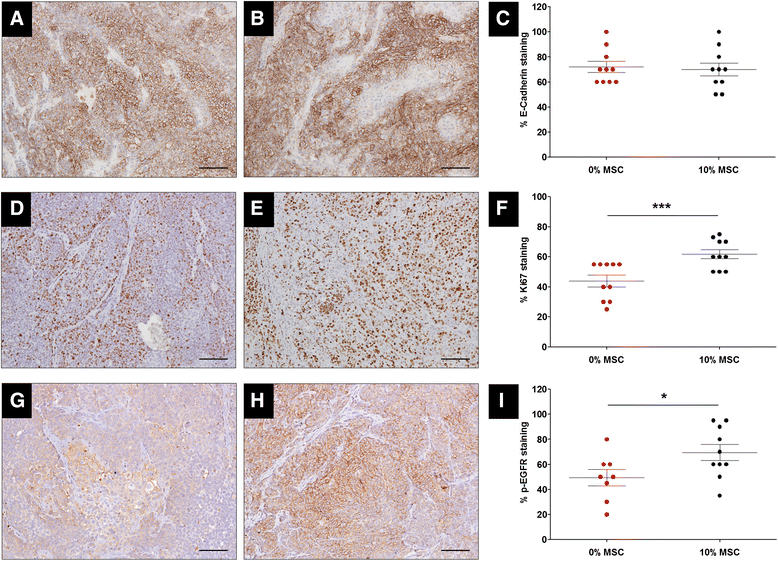
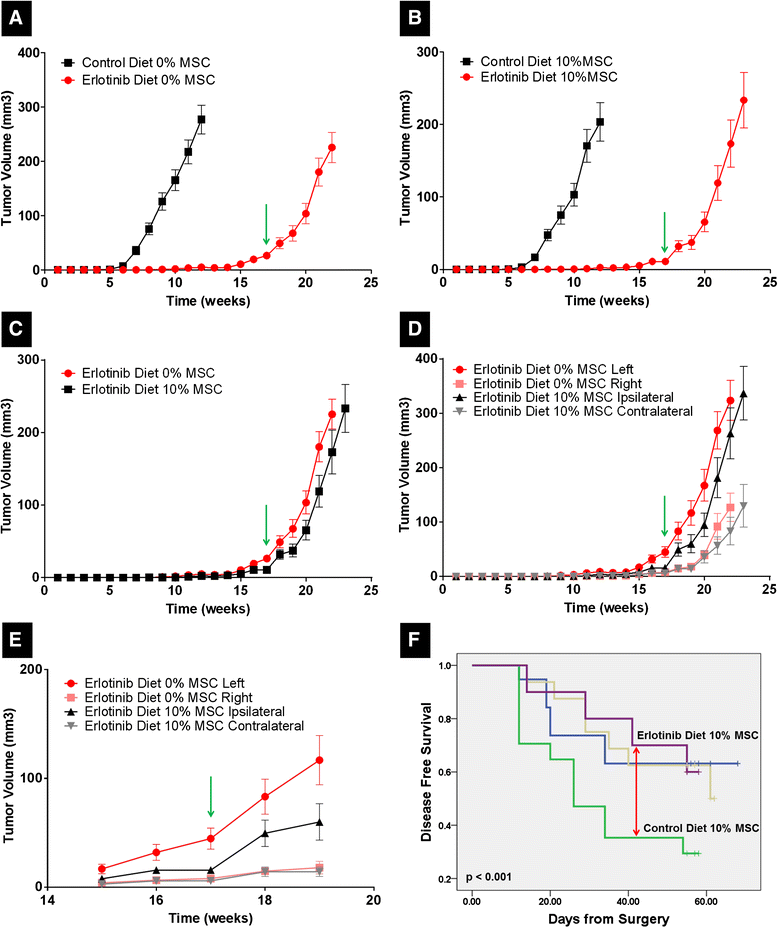
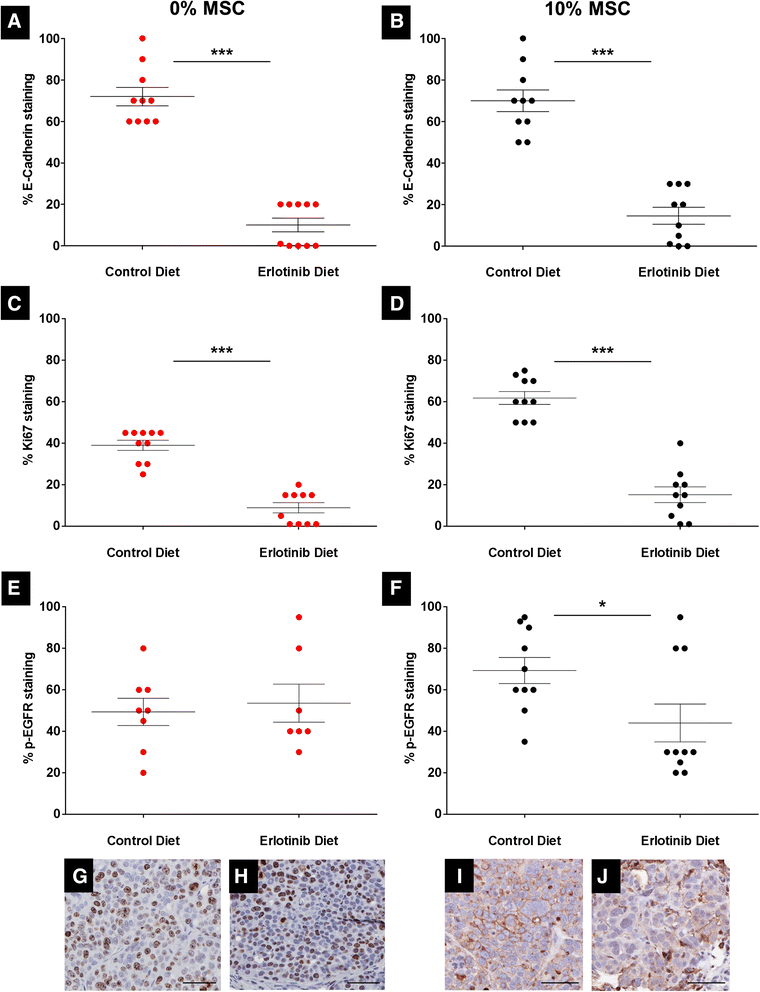
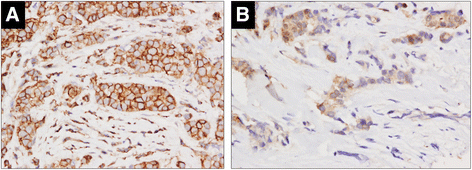
Results: MSCs co-injection significantly increased the clinical features of skin invasion and metastasis in the SUM149 xenograft model. Primary tumors co-injected with MSCs expressed higher phospho-epidermal growth factor receptor (p-EGFR) and promoted metastasis development after tumor resection, effects that were abrogated by treatment with the epidermal growth factor receptor (EGFR) inhibitor, erlotinib. E-cadherin expression was maintained in primary tumor xenografts with MSCs co-injection compared to control and erlotinib treatment dramatically decreased this expression in control and MSCs co-injected tumors. Tumor samples from patients demonstrate correlation between stromal and tumor p-EGFR staining only in IBC tumors.
Conclusions: Our findings demonstrate that the IBC clinical phenotype is promoted by signaling from the microenvironment perhaps in addition to tumor cell drivers.
Figures

Similar articles
-
Mesenchymal stem cells and macrophages interact through IL-6 to promote inflammatory breast cancer in pre-clinical models.Oncotarget. 2016 Dec 13;7(50):82482-82492. doi: 10.18632/oncotarget.12694. Oncotarget. 2016. PMID: 27756885 Free PMC article.
-
Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways.Mol Cancer. 2017 Mar 7;16(1):57. doi: 10.1186/s12943-017-0621-z. Mol Cancer. 2017. PMID: 28270211 Free PMC article.
-
Epidermal growth factor receptor tyrosine kinase inhibitor reverses mesenchymal to epithelial phenotype and inhibits metastasis in inflammatory breast cancer.Clin Cancer Res. 2009 Nov 1;15(21):6639-48. doi: 10.1158/1078-0432.CCR-09-0951. Epub 2009 Oct 13. Clin Cancer Res. 2009. PMID: 19825949 Free PMC article.
-
Deciphering the role of interferon alpha signaling and microenvironment crosstalk in inflammatory breast cancer.Breast Cancer Res. 2019 May 6;21(1):59. doi: 10.1186/s13058-019-1140-1. Breast Cancer Res. 2019. PMID: 31060575 Free PMC article. Review.
-
Epigenetics in Inflammatory Breast Cancer: Biological Features and Therapeutic Perspectives.Cells. 2020 May 8;9(5):1164. doi: 10.3390/cells9051164. Cells. 2020. PMID: 32397183 Free PMC article. Review.
Cited by
-
EGFR signaling promotes inflammation and cancer stem-like activity in inflammatory breast cancer.Oncotarget. 2017 Jul 4;8(40):67904-67917. doi: 10.18632/oncotarget.18958. eCollection 2017 Sep 15. Oncotarget. 2017. PMID: 28978083 Free PMC article.
-
IL10-modified Human Mesenchymal Stem Cells inhibit Pancreatic Cancer growth through Angiogenesis Inhibition.J Cancer. 2020 Jul 9;11(18):5345-5352. doi: 10.7150/jca.38062. eCollection 2020. J Cancer. 2020. PMID: 32742480 Free PMC article.
-
Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine.Stem Cells Transl Med. 2017 Dec;6(12):2173-2185. doi: 10.1002/sctm.17-0129. Epub 2017 Oct 26. Stem Cells Transl Med. 2017. PMID: 29076267 Free PMC article. Review.
-
Mesenchymal stem cells: key players in cancer progression.Mol Cancer. 2017 Feb 1;16(1):31. doi: 10.1186/s12943-017-0597-8. Mol Cancer. 2017. PMID: 28148268 Free PMC article. Review.
-
Histone deacetylase inhibitors reinforce the phenotypical markers of breast epithelial or mesenchymal cancer cells but inhibit their migratory properties.Cancer Manag Res. 2019 Sep 13;11:8345-8358. doi: 10.2147/CMAR.S210029. eCollection 2019. Cancer Manag Res. 2019. PMID: 31571991 Free PMC article.
References
-
- Van Laere SJ, Ueno NT, Finetti P, Vermeulen PB, Lucci A, Robertson FM, et al. Uncovering the molecular secrets of Inflammatory Breast Cancer biology: An integrated analysis of three distinct Affymetrix gene expression data sets. Clin Cancer Res. 2013;19:4685–4696. doi: 10.1158/1078-0432.CCR-12-2549. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

