Prematurity and respiratory outcomes program (PROP): study protocol of a prospective multicenter study of respiratory outcomes of preterm infants in the United States
- PMID: 25886363
- PMCID: PMC4407843
- DOI: 10.1186/s12887-015-0346-3
Prematurity and respiratory outcomes program (PROP): study protocol of a prospective multicenter study of respiratory outcomes of preterm infants in the United States
Abstract
Background: With improved survival rates, short- and long-term respiratory complications of premature birth are increasing, adding significantly to financial and health burdens in the United States. In response, in May 2010, the National Institutes of Health (NIH) and the National Heart, Lung, and Blood Institute (NHLBI) funded a 5-year $18.5 million research initiative to ultimately improve strategies for managing the respiratory complications of preterm and low birth weight infants. Using a collaborative, multi-disciplinary structure, the resulting Prematurity and Respiratory Outcomes Program (PROP) seeks to understand factors that correlate with future risk for respiratory morbidity.
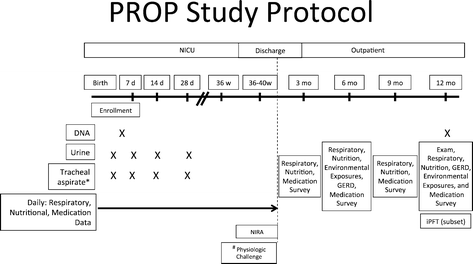
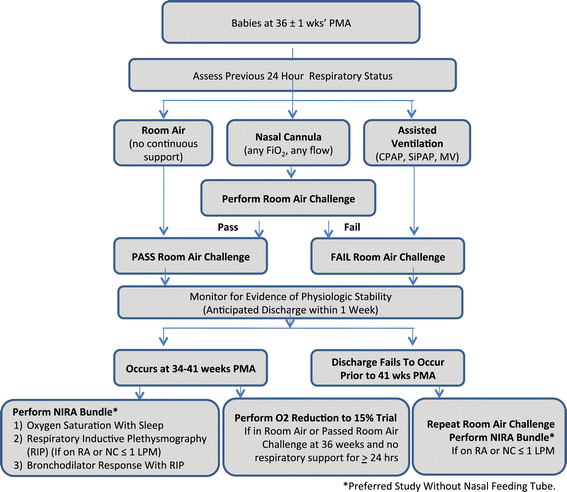
Methods/design: The PROP is an observational prospective cohort study performed by a consortium of six clinical centers (incorporating tertiary neonatal intensive care units [NICU] at 13 sites) and a data-coordinating center working in collaboration with the NHLBI. Each clinical center contributes subjects to the study, enrolling infants with gestational ages 23 0/7 to 28 6/7 weeks with an anticipated target of 750 survivors at 36 weeks post-menstrual age. In addition, each center brings specific areas of scientific focus to the Program. The primary study hypothesis is that in survivors of extreme prematurity specific biologic, physiologic and clinical data predicts respiratory morbidity between discharge and 1 year corrected age. Analytic statistical methodology includes model-based and non-model-based analyses, descriptive analyses and generalized linear mixed models.
Discussion: PROP incorporates aspects of NICU care to develop objective biomarkers and outcome measures of respiratory morbidity in the <29 week gestation population beyond just the NICU hospitalization, thereby leading to novel understanding of the nature and natural history of neonatal lung disease and of potential mechanistic and therapeutic targets in at-risk subjects.
Trial registration: Clinical Trials.gov NCT01435187.
Figures


Similar articles
-
Extreme Prematurity and Pulmonary Outcomes Program in Saitama: Protocol for a Prospective Multicenter Cohort Study in Japan.JMIR Res Protoc. 2021 Mar 5;10(3):e22948. doi: 10.2196/22948. JMIR Res Protoc. 2021. PMID: 33666556 Free PMC article.
-
Respiratory medication use in extremely premature (<29 weeks) infants during initial NICU hospitalization: Results from the prematurity and respiratory outcomes program.Pediatr Pulmonol. 2020 Feb;55(2):360-368. doi: 10.1002/ppul.24592. Epub 2019 Dec 3. Pediatr Pulmonol. 2020. PMID: 31794157
-
A common problem for neonatal intensive care units: late preterm infants, a prospective study with term controls in a large perinatal center.J Matern Fetal Neonatal Med. 2013 Mar;26(5):459-62. doi: 10.3109/14767058.2012.735994. Epub 2012 Oct 31. J Matern Fetal Neonatal Med. 2013. PMID: 23106478
-
Populations at risk for developing respiratory syncytial virus and risk factors for respiratory syncytial virus severity: infants with predisposing conditions.Pediatr Infect Dis J. 2003 Feb;22(2 Suppl):S33-7; discussion S37-9. doi: 10.1097/01.inf.0000053883.08663.e5. Pediatr Infect Dis J. 2003. PMID: 12671450 Review.
-
Early NICU discharge of very low birth weight infants: a critical review and analysis.Semin Neonatol. 2003 Apr;8(2):95-115. doi: 10.1016/S1084-2756(02)00219-1. Semin Neonatol. 2003. PMID: 15001147 Review.
Cited by
-
Long-term pulmonary outcomes in BPD throughout the life-course.J Perinatol. 2024 Apr 3. doi: 10.1038/s41372-024-01957-9. Online ahead of print. J Perinatol. 2024. PMID: 38570594 Review.
-
Phthalate exposure in the neonatal intensive care unit is associated with development of bronchopulmonary dysplasia.Environ Int. 2023 Aug;178:108117. doi: 10.1016/j.envint.2023.108117. Epub 2023 Jul 26. Environ Int. 2023. PMID: 37517179 Free PMC article.
-
Pre-Flight Hypoxemia Challenge Testing in Bronchopulmonary Dysplasia.Pediatrics. 2023 Aug 1;152(2):e2022061001. doi: 10.1542/peds.2022-061001. Pediatrics. 2023. PMID: 37503557 Free PMC article.
-
Environmental influences on child health outcomes: cohorts of individuals born very preterm.Pediatr Res. 2023 Apr;93(5):1161-1176. doi: 10.1038/s41390-022-02230-5. Epub 2022 Aug 10. Pediatr Res. 2023. PMID: 35948605 Free PMC article. Review.
-
Respiratory Follow Up of the Premature Neonates-Rationale and Practical Issues.J Clin Med. 2022 Mar 21;11(6):1746. doi: 10.3390/jcm11061746. J Clin Med. 2022. PMID: 35330070 Free PMC article. Review.
References
-
- Martinez FD, Morgan WJ, Wright AL, Holberg C, Taussig LM. Initial airway function is a risk factor for recurrent wheezing respiratory illnesses during the first three years of life. Group Health Medical Associates. Am Rev Respir Dis. 1991;143(2):312–316. doi: 10.1164/ajrccm/143.2.312. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

