Grapefruit-Derived Nanovectors Use an Activated Leukocyte Trafficking Pathway to Deliver Therapeutic Agents to Inflammatory Tumor Sites
- PMID: 25883092
- PMCID: PMC4470740
- DOI: 10.1158/0008-5472.CAN-14-3095
Grapefruit-Derived Nanovectors Use an Activated Leukocyte Trafficking Pathway to Deliver Therapeutic Agents to Inflammatory Tumor Sites
Erratum in
-
Correction: Grapefruit-Derived Nanovectors Use an Activated Leukocyte Trafficking Pathway to Deliver Therapeutic Agents to Inflammatory Tumor Sites.Cancer Res. 2016 May 1;76(9):2845. doi: 10.1158/0008-5472.CAN-16-0564. Cancer Res. 2016. PMID: 27197268 No abstract available.
Abstract
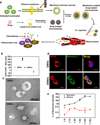
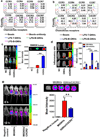
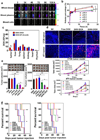
Inflammation is a hallmark of cancer. Activated immune cells are intrinsically capable of homing to inflammatory sites. Using three inflammatory-driven disease mouse models, we show that grapefruit-derived nanovectors (GNV) coated with inflammatory-related receptor enriched membranes of activated leukocytes (IGNVs) are enhanced for homing to inflammatory tumor tissues. Blocking LFA-1 or CXCR1 and CXCR2 on the IGNVs significantly inhibits IGNV homing to the inflammatory tissue. The therapeutic potential of IGNVs was further demonstrated by enhancing the chemotherapeutic effect as shown by inhibition of tumor growth in two tumor models and inhibiting the inflammatory effects of dextran sulfate sodium-induced mouse colitis. The fact that IGNVs are capable of homing to inflammatory tissue and that chemokines are overexpressed in diseased human tissue provides the rationale for using IGNVs to more directly deliver therapeutic agents to inflammatory tumor sites and the rationale for the use of IGNVs as treatment for certain cancers in personalized medicine.
©2015 American Association for Cancer Research.
Conflict of interest statement
CONFLICT OF INTEREST: No potential conflicts of interest were disclosed.
Figures


Similar articles
-
Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids.Nat Commun. 2013;4:1867. doi: 10.1038/ncomms2886. Nat Commun. 2013. PMID: 23695661 Free PMC article.
-
Grapefruit-derived Nanovectors Delivering Therapeutic miR17 Through an Intranasal Route Inhibit Brain Tumor Progression.Mol Ther. 2016 Feb;24(1):96-105. doi: 10.1038/mt.2015.188. Epub 2015 Oct 7. Mol Ther. 2016. PMID: 26444082 Free PMC article.
-
Inflammatory chemokines and metastasis--tracing the accessory.Oncogene. 2014 Jun 19;33(25):3217-24. doi: 10.1038/onc.2013.272. Epub 2013 Jul 15. Oncogene. 2014. PMID: 23851506 Review.
-
Potent effector function of tumor-sensitized L-selectin(low) T cells against subcutaneous tumors requires LFA-1 co-stimulation.Otolaryngol Head Neck Surg. 2001 Apr;124(4):436-41. doi: 10.1067/mhn.2001.114253. Otolaryngol Head Neck Surg. 2001. PMID: 11283503
-
Key role of chemokines and chemokine receptors in inflammation, immunity, neoplasia, and infectious disease.Vet Pathol. 1999 Sep;36(5):357-67. doi: 10.1354/vp.36-5-357. Vet Pathol. 1999. PMID: 10490203 Review.
Cited by
-
Delivery strategies for macromolecular drugs in cancer therapy.Acta Pharm Sin B. 2020 Jun;10(6):979-986. doi: 10.1016/j.apsb.2020.01.009. Epub 2020 Jan 26. Acta Pharm Sin B. 2020. PMID: 32642406 Free PMC article. Review.
-
Exploring the Biomedical Frontiers of Plant-Derived Nanoparticles: Synthesis and Biological Reactions.Pharmaceutics. 2024 Jul 11;16(7):923. doi: 10.3390/pharmaceutics16070923. Pharmaceutics. 2024. PMID: 39065620 Free PMC article. Review.
-
Extracellular Vesicles as Drug Delivery System for Cancer Therapy.Pharmaceutics. 2024 Aug 1;16(8):1029. doi: 10.3390/pharmaceutics16081029. Pharmaceutics. 2024. PMID: 39204374 Free PMC article. Review.
-
A mini-review: Advances in plant-derived extracellular vesicles as nano-delivery systems for tumour therapy.Front Bioeng Biotechnol. 2022 Dec 15;10:1076348. doi: 10.3389/fbioe.2022.1076348. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36588940 Free PMC article. Review.
-
Emerging Nano-/Microapproaches for Cancer Immunotherapy.Adv Sci (Weinh). 2019 Jan 13;6(6):1801847. doi: 10.1002/advs.201801847. eCollection 2019 Mar 20. Adv Sci (Weinh). 2019. PMID: 30937265 Free PMC article. Review.
References
-
- Kugelberg E. T cell responses: kiss and run. Nature reviews Immunology. 2014;14(3):134. - PubMed
-
- Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nature reviews Immunology. 2007;7(10):803–815. - PubMed
-
- Schall TJ, Proudfoot AE. Overcoming hurdles in developing successful drugs targeting chemokine receptors. Nature reviews Immunology. 2011;11(5):355–363. - PubMed
-
- Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nature reviews Immunology. 2006;6(12):907–918. - PubMed
-
- Ben-Baruch A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clinical & experimental metastasis. 2008;25(4):345–356. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

