Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering
- PMID: 25880725
- PMCID: PMC4608855
- DOI: 10.1002/adhm.201500005
Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering
Abstract
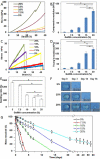
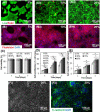
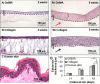
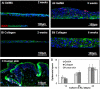
Natural hydrogels are promising scaffolds to engineer epidermis. Currently, natural hydrogels used to support epidermal regeneration are mainly collagen- or gelatin-based, which mimic the natural dermal extracellular matrix but often suffer from insufficient and uncontrollable mechanical and degradation properties. In this study, a photocrosslinkable gelatin (i.e., gelatin methacrylamide (GelMA)) with tunable mechanical, degradation, and biological properties is used to engineer the epidermis for skin tissue engineering applications. The results reveal that the mechanical and degradation properties of the developed hydrogels can be readily modified by varying the hydrogel concentration, with elastic and compressive moduli tuned from a few kPa to a few hundred kPa, and the degradation times varied from a few days to several months. Additionally, hydrogels of all concentrations displayed excellent cell viability (>90%) with increasing cell adhesion and proliferation corresponding to increases in hydrogel concentrations. Furthermore, the hydrogels are found to support keratinocyte growth, differentiation, and stratification into a reconstructed multilayered epidermis with adequate barrier functions. The robust and tunable properties of GelMA hydrogels suggest that the keratinocyte laden hydrogels can be used as epidermal substitutes, wound dressings, or substrates to construct various in vitro skin models.
Keywords: degradation; epidermis; keratinocytes; mechanical properties; photocrosslinkable gelatin.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

Similar articles
-
Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing.Acta Biomater. 2017 Feb;49:66-77. doi: 10.1016/j.actbio.2016.11.017. Epub 2016 Nov 5. Acta Biomater. 2017. PMID: 27826004 Free PMC article.
-
Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs.Macromol Biosci. 2013 May;13(5):551-61. doi: 10.1002/mabi.201200471. Epub 2013 Feb 18. Macromol Biosci. 2013. PMID: 23420700
-
Multilayered polycaprolactone/gelatin fiber-hydrogel composite for tendon tissue engineering.Acta Biomater. 2016 Apr 15;35:68-76. doi: 10.1016/j.actbio.2016.03.004. Epub 2016 Mar 2. Acta Biomater. 2016. PMID: 26945631 Free PMC article.
-
Recent trends in gelatin methacryloyl nanocomposite hydrogels for tissue engineering.J Biomed Mater Res A. 2022 Mar;110(3):708-724. doi: 10.1002/jbm.a.37310. Epub 2021 Sep 24. J Biomed Mater Res A. 2022. PMID: 34558808 Review.
-
Bioengineering for vascularization: Trends and directions of photocrosslinkable gelatin methacrylate hydrogels.Front Bioeng Biotechnol. 2022 Nov 17;10:1053491. doi: 10.3389/fbioe.2022.1053491. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36466323 Free PMC article. Review.
Cited by
-
Effects of Gelatin Methacrylate Bio-ink Concentration on Mechano-Physical Properties and Human Dermal Fibroblast Behavior.Polymers (Basel). 2020 Aug 26;12(9):1930. doi: 10.3390/polym12091930. Polymers (Basel). 2020. PMID: 32859028 Free PMC article.
-
Recombinant Human Collagen-Based Bioinks for the 3D Bioprinting of Full-thickness Human Skin Equivalent.Int J Bioprint. 2022 Aug 25;8(4):611. doi: 10.18063/ijb.v8i4.611. eCollection 2022. Int J Bioprint. 2022. PMID: 36404779 Free PMC article.
-
Stimulus-Responsive Hydrogels as Drug Delivery Systems for Inflammation Targeted Therapy.Adv Sci (Weinh). 2024 Jan;11(1):e2306152. doi: 10.1002/advs.202306152. Epub 2023 Nov 20. Adv Sci (Weinh). 2024. PMID: 37985923 Free PMC article. Review.
-
Recent advances in engineering hydrogels for niche biomimicking and hematopoietic stem cell culturing.Front Bioeng Biotechnol. 2022 Nov 24;10:1049965. doi: 10.3389/fbioe.2022.1049965. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36507253 Free PMC article. Review.
-
Accelerate Healing of Severe Burn Wounds by Mouse Bone Marrow Mesenchymal Stem Cell-Seeded Biodegradable Hydrogel Scaffold Synthesized from Arginine-Based Poly(ester amide) and Chitosan.Stem Cells Dev. 2018 Dec 1;27(23):1605-1620. doi: 10.1089/scd.2018.0106. Epub 2018 Oct 23. Stem Cells Dev. 2018. PMID: 30215325 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- AR063745/AR/NIAMS NIH HHS/United States
- R01 EB012597/EB/NIBIB NIH HHS/United States
- R56 AI105024/AI/NIAID NIH HHS/United States
- EB012597/EB/NIBIB NIH HHS/United States
- R56 AR063745/AR/NIAMS NIH HHS/United States
- AI105024/AI/NIAID NIH HHS/United States
- BB/H011293/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- DE021468/DE/NIDCR NIH HHS/United States
- R01 DE021468/DE/NIDCR NIH HHS/United States
- HL099073/HL/NHLBI NIH HHS/United States
- R01 AR057837/AR/NIAMS NIH HHS/United States
- R01 HL099073/HL/NHLBI NIH HHS/United States
- AR057837/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

