Mitofusin 2 ablation increases endoplasmic reticulum-mitochondria coupling
- PMID: 25870285
- PMCID: PMC4418914
- DOI: 10.1073/pnas.1504880112
Mitofusin 2 ablation increases endoplasmic reticulum-mitochondria coupling
Abstract
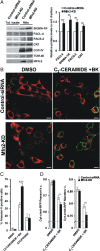
The organization and mutual interactions between endoplasmic reticulum (ER) and mitochondria modulate key aspects of cell pathophysiology. Several proteins have been suggested to be involved in keeping ER and mitochondria at a correct distance. Among them, in mammalian cells, mitofusin 2 (Mfn2), located on both the outer mitochondrial membrane and the ER surface, has been proposed to be a physical tether between the two organelles, forming homotypic interactions and heterocomplexes with its homolog Mfn1. Recently, this widely accepted model has been challenged using quantitative EM analysis. Using a multiplicity of morphological, biochemical, functional, and genetic approaches, we demonstrate that Mfn2 ablation increases the structural and functional ER-mitochondria coupling. In particular, we show that in different cell types Mfn2 ablation or silencing increases the close contacts between the two organelles and strengthens the efficacy of inositol trisphosphate (IP3)-induced Ca(2+) transfer from the ER to mitochondria, sensitizing cells to a mitochondrial Ca(2+) overload-dependent death. We also show that the previously reported discrepancy between electron and fluorescence microscopy data on ER-mitochondria proximity in Mfn2-ablated cells is only apparent. By using a different type of morphological analysis of fluorescent images that takes into account (and corrects for) the gross modifications in mitochondrial shape resulting from Mfn2 ablation, we demonstrate that an increased proximity between the organelles is also observed by confocal microscopy when Mfn2 levels are reduced. Based on these results, we propose a new model for ER-mitochondria juxtaposition in which Mfn2 works as a tethering antagonist preventing an excessive, potentially toxic, proximity between the two organelles.
Keywords: ER–mitochondria tethering; inter-organellar communication; mitofusin 2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Splice variants of mitofusin 2 shape the endoplasmic reticulum and tether it to mitochondria.Science. 2023 Jun 23;380(6651):eadh9351. doi: 10.1126/science.adh9351. Epub 2023 Jun 23. Science. 2023. PMID: 37347868
-
Presenilin 2 Modulates Endoplasmic Reticulum-Mitochondria Coupling by Tuning the Antagonistic Effect of Mitofusin 2.Cell Rep. 2016 Jun 7;15(10):2226-2238. doi: 10.1016/j.celrep.2016.05.013. Epub 2016 May 26. Cell Rep. 2016. PMID: 27239030
-
Mitofusin 2 tethers endoplasmic reticulum to mitochondria.Nature. 2008 Dec 4;456(7222):605-10. doi: 10.1038/nature07534. Nature. 2008. PMID: 19052620
-
Functional implications of mitofusin 2-mediated mitochondrial-SR tethering.J Mol Cell Cardiol. 2015 Jan;78:123-8. doi: 10.1016/j.yjmcc.2014.09.015. Epub 2014 Sep 22. J Mol Cell Cardiol. 2015. PMID: 25252175 Free PMC article. Review.
-
Highlighting the endoplasmic reticulum-mitochondria connection: Focus on Mitofusin 2.Pharmacol Res. 2018 Feb;128:42-51. doi: 10.1016/j.phrs.2018.01.003. Epub 2018 Jan 5. Pharmacol Res. 2018. PMID: 29309902 Review.
Cited by
-
Mitochondria-Associated Endoplasmic Reticulum Membranes: Inextricably Linked with Autophagy Process.Oxid Med Cell Longev. 2022 Aug 23;2022:7086807. doi: 10.1155/2022/7086807. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36052160 Free PMC article. Review.
-
The role of Mfn2 in the structure and function of endoplasmic reticulum-mitochondrial tethering in vivo.J Cell Sci. 2021 Jul 1;134(13):jcs253443. doi: 10.1242/jcs.253443. Epub 2021 Jul 9. J Cell Sci. 2021. PMID: 34110411 Free PMC article.
-
Raft-like lipid microdomains drive autophagy initiation via AMBRA1-ERLIN1 molecular association within MAMs.Autophagy. 2021 Sep;17(9):2528-2548. doi: 10.1080/15548627.2020.1834207. Epub 2020 Oct 23. Autophagy. 2021. PMID: 33034545 Free PMC article.
-
Mul1 restrains Parkin-mediated mitophagy in mature neurons by maintaining ER-mitochondrial contacts.Nat Commun. 2019 Aug 13;10(1):3645. doi: 10.1038/s41467-019-11636-5. Nat Commun. 2019. PMID: 31409786 Free PMC article.
-
The Charcot-Marie Tooth Disease Mutation R94Q in MFN2 Decreases ATP Production but Increases Mitochondrial Respiration under Conditions of Mild Oxidative Stress.Cells. 2019 Oct 21;8(10):1289. doi: 10.3390/cells8101289. Cells. 2019. PMID: 31640251 Free PMC article.
References
-
- Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;265(13):7248–7256. - PubMed
-
- Rusiñol AE, Cui Z, Chen MH, Vance JE. A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. J Biol Chem. 1994;269(44):27494–27502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

