TRAF Family Member-associated NF-κB Activator (TANK) Inhibits Genotoxic Nuclear Factor κB Activation by Facilitating Deubiquitinase USP10-dependent Deubiquitination of TRAF6 Ligase
- PMID: 25861989
- PMCID: PMC4505586
- DOI: 10.1074/jbc.M115.643767
TRAF Family Member-associated NF-κB Activator (TANK) Inhibits Genotoxic Nuclear Factor κB Activation by Facilitating Deubiquitinase USP10-dependent Deubiquitination of TRAF6 Ligase
Abstract
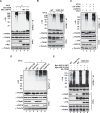
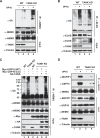
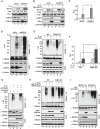
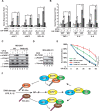
DNA damage-induced NF-κB activation plays a critical role in regulating cellular response to genotoxic stress. However, the molecular mechanisms controlling the magnitude and duration of this genotoxic NF-κB signaling cascade are poorly understood. We recently demonstrated that genotoxic NF-κB activation is regulated by reversible ubiquitination of several essential mediators involved in this signaling pathway. Here we show that TRAF family member-associated NF-κB activator (TANK) negatively regulates NF-κB activation by DNA damage via inhibiting ubiquitination of TRAF6. Despite the lack of a deubiquitination enzyme domain, TANK has been shown to negatively regulate the ubiquitination of TRAF proteins. We found TANK formed a complex with MCPIP1 (also known as ZC3H12A) and a deubiquitinase, USP10, which was essential for the USP10-dependent deubiquitination of TRAF6 and the resolution of genotoxic NF-κB activation upon DNA damage. Clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9-mediated deletion of TANK in human cells significantly enhanced NF-κB activation by genotoxic treatment, resulting in enhanced cell survival and increased inflammatory cytokine production. Furthermore, we found that the TANK-MCPIP1-USP10 complex also decreased TRAF6 ubiquitination in cells treated with IL-1β or LPS. In accordance, depletion of USP10 enhanced NF-κB activation induced by IL-1β or LPS. Collectively, our data demonstrate that TANK serves as an important negative regulator of NF-κB signaling cascades induced by genotoxic stress and IL-1R/Toll-like receptor stimulation in a manner dependent on MCPIP1/USP10-mediated TRAF6 deubiquitination.
Keywords: DNA damage; NF-κB; TANK; TNF receptor-associated factor (TRAF); ubiquitin-dependent protease; ubiquitylation (ubiquitination).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
USP10 inhibits genotoxic NF-κB activation by MCPIP1-facilitated deubiquitination of NEMO.EMBO J. 2013 Dec 11;32(24):3206-19. doi: 10.1038/emboj.2013.247. Epub 2013 Nov 22. EMBO J. 2013. PMID: 24270572 Free PMC article.
-
TRAF family member-associated NF-κB activator (TANK) is a negative regulator of osteoclastogenesis and bone formation.J Biol Chem. 2012 Aug 17;287(34):29114-24. doi: 10.1074/jbc.M112.347799. Epub 2012 Jul 6. J Biol Chem. 2012. PMID: 22773835 Free PMC article.
-
Ubiquitin-specific protease 4 (USP4) targets TRAF2 and TRAF6 for deubiquitination and inhibits TNFα-induced cancer cell migration.Biochem J. 2012 Feb 1;441(3):979-86. doi: 10.1042/BJ20111358. Biochem J. 2012. PMID: 22029577
-
TNF receptor-associated factor 6 (TRAF6) plays crucial roles in multiple biological systems through polyubiquitination-mediated NF-κB activation.Proc Jpn Acad Ser B Phys Biol Sci. 2021;97(4):145-160. doi: 10.2183/pjab.97.009. Proc Jpn Acad Ser B Phys Biol Sci. 2021. PMID: 33840674 Free PMC article. Review.
-
The role of K63-linked polyubiquitination in cardiac hypertrophy.J Cell Mol Med. 2018 Oct;22(10):4558-4567. doi: 10.1111/jcmm.13669. Epub 2018 Aug 13. J Cell Mol Med. 2018. PMID: 30102008 Free PMC article. Review.
Cited by
-
MCPIP1 ribonuclease can bind and cleave AURKA mRNA in MYCN-amplified neuroblastoma cells.RNA Biol. 2021 Jan;18(1):144-156. doi: 10.1080/15476286.2020.1804698. Epub 2020 Aug 20. RNA Biol. 2021. PMID: 32757706 Free PMC article.
-
Corrigendum: gga-miR-155 Enhances Type I Interferon Expression and Suppresses Infectious Burse Disease Virus Replication via Targeting SOCS1 and TANK.Front Cell Infect Microbiol. 2020 Jul 15;10:324. doi: 10.3389/fcimb.2020.00324. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32760677 Free PMC article.
-
Penalized negative binomial models for modeling an overdispersed count outcome with a high-dimensional predictor space: Application predicting micronuclei frequency.PLoS One. 2019 Jan 8;14(1):e0209923. doi: 10.1371/journal.pone.0209923. eCollection 2019. PLoS One. 2019. PMID: 30620740 Free PMC article.
-
Caprin-1 binding to the critical stress granule protein G3BP1 is influenced by pH.Open Biol. 2023 May;13(5):220369. doi: 10.1098/rsob.220369. Epub 2023 May 10. Open Biol. 2023. PMID: 37161291 Free PMC article.
-
Regnase-1, a rapid response ribonuclease regulating inflammation and stress responses.Cell Mol Immunol. 2017 May;14(5):412-422. doi: 10.1038/cmi.2016.70. Epub 2017 Feb 13. Cell Mol Immunol. 2017. PMID: 28194024 Free PMC article. Review.
References
-
- Baldwin A. S. (2012) Regulation of cell death and autophagy by IKK and NF-κB: critical mechanisms in immune function and cancer. Immunol. Rev. 246, 327–345 - PubMed
-
- Wang C. Y., Mayo M. W., Korneluk R. G., Goeddel D. V., Baldwin A. S., Jr. (1998) NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281, 1680–1683 - PubMed
-
- Nakanishi C., Toi M. (2005) Nuclear factor-κB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer 5, 297–309 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

