Stress responses. Mutations in a translation initiation factor identify the target of a memory-enhancing compound
- PMID: 25858979
- PMCID: PMC4538794
- DOI: 10.1126/science.aaa6986
Stress responses. Mutations in a translation initiation factor identify the target of a memory-enhancing compound
Abstract
The integrated stress response (ISR) modulates messenger RNA translation to regulate the mammalian unfolded protein response (UPR), immunity, and memory formation. A chemical ISR inhibitor, ISRIB, enhances cognitive function and modulates the UPR in vivo. To explore mechanisms involved in ISRIB action, we screened cultured mammalian cells for somatic mutations that reversed its effect on the ISR. Clustered missense mutations were found at the amino-terminal portion of the delta subunit of guanine nucleotide exchange factor (GEF) eIF2B. When reintroduced by CRISPR-Cas9 gene editing of wild-type cells, these mutations reversed both ISRIB-mediated inhibition of the ISR and its stimulatory effect on eIF2B GEF activity toward its substrate, the translation initiation factor eIF2, in vitro. Thus, ISRIB targets an interaction between eIF2 and eIF2B that lies at the core of the ISR.
Copyright © 2015, American Association for the Advancement of Science.
Figures
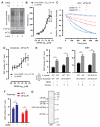
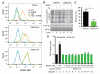
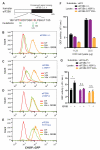
Comment in
-
Cell biology. Blocking stress response for better memory?Science. 2015 May 29;348(6238):967-8. doi: 10.1126/science.aac4832. Science. 2015. PMID: 26023119 No abstract available.
Similar articles
-
Cell biology. Blocking stress response for better memory?Science. 2015 May 29;348(6238):967-8. doi: 10.1126/science.aac4832. Science. 2015. PMID: 26023119 No abstract available.
-
ISRIB Blunts the Integrated Stress Response by Allosterically Antagonising the Inhibitory Effect of Phosphorylated eIF2 on eIF2B.Mol Cell. 2021 Jan 7;81(1):88-103.e6. doi: 10.1016/j.molcel.2020.10.031. Epub 2020 Nov 20. Mol Cell. 2021. PMID: 33220178 Free PMC article.
-
Structure of the nucleotide exchange factor eIF2B reveals mechanism of memory-enhancing molecule.Science. 2018 Mar 30;359(6383):eaaq0939. doi: 10.1126/science.aaq0939. Science. 2018. PMID: 29599213 Free PMC article.
-
Structural insights into ISRIB, a memory-enhancing inhibitor of the integrated stress response.FEBS J. 2020 Jan;287(2):239-245. doi: 10.1111/febs.15073. Epub 2019 Nov 7. FEBS J. 2020. PMID: 31550413 Review.
-
Surviving and Adapting to Stress: Translational Control and the Integrated Stress Response.Antioxid Redox Signal. 2023 Aug;39(4-6):351-373. doi: 10.1089/ars.2022.0123. Epub 2023 May 9. Antioxid Redox Signal. 2023. PMID: 36943285 Free PMC article. Review.
Cited by
-
Inhibition of the integrated stress response by viral proteins that block p-eIF2-eIF2B association.Nat Microbiol. 2020 Nov;5(11):1361-1373. doi: 10.1038/s41564-020-0759-0. Epub 2020 Jul 20. Nat Microbiol. 2020. PMID: 32690955
-
eIF2β is critical for eIF5-mediated GDP-dissociation inhibitor activity and translational control.Nucleic Acids Res. 2016 Nov 16;44(20):9698-9709. doi: 10.1093/nar/gkw657. Epub 2016 Jul 25. Nucleic Acids Res. 2016. PMID: 27458202 Free PMC article.
-
Presynaptic Protein Synthesis Is Required for Long-Term Plasticity of GABA Release.Neuron. 2016 Oct 19;92(2):479-492. doi: 10.1016/j.neuron.2016.09.040. Neuron. 2016. PMID: 27764673 Free PMC article.
-
Small molecule strategies to harness the unfolded protein response: where do we go from here?J Biol Chem. 2020 Nov 13;295(46):15692-15711. doi: 10.1074/jbc.REV120.010218. Epub 2020 Sep 4. J Biol Chem. 2020. PMID: 32887796 Free PMC article. Review.
-
Herpesviruses and the Unfolded Protein Response.Viruses. 2019 Dec 21;12(1):17. doi: 10.3390/v12010017. Viruses. 2019. PMID: 31877732 Free PMC article. Review.
References
-
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11:619–633. - PubMed
-
- Harding H, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell. 2000;5:897–904. - PubMed
-
- Costa-Mattioli M, Gobert D, Harding HP, Herdy B, Azzi M, Bruno M, Ben Mamou C, Marcinkiewicz E, Yoshida M, Imataka H, Cuello AC, Seidah N, Sossin W, Lacaille J-C, Ron D, Nader K, Sonenberg N. Translational control of hippocampal synaptic plasticity and memory by an eIF2 kinase, GCN2. Nature. 2005;436:1166–1173. - PMC - PubMed
-
- Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 Kinase in T Cells Mediates Proliferative Arrest and Anergy Induction in Response to Indoleamine 2,3-Dioxygenase. Immunity. 2005;22:633–642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

