The prostaglandin D₂ receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung
- PMID: 25850654
- PMCID: PMC4598246
- DOI: 10.1038/mi.2015.21
The prostaglandin D₂ receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung
Abstract
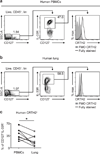
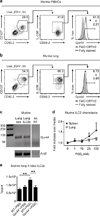
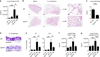
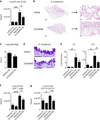
Group 2 innate lymphoid cells (ILC2s) promote type 2 cytokine-dependent immunity, inflammation, and tissue repair. Although epithelial cell-derived cytokines regulate ILC2 effector functions, the pathways that control the in vivo migration of ILC2s into inflamed tissues remain poorly understood. Here, we provide the first demonstration that expression of the prostaglandin D2 (PGD2) receptor CRTH2 (chemoattractant receptor-homologous molecule expressed on Th2 cells) regulates the in vivo accumulation of ILC2s in the lung. Although a significant proportion of ILC2s isolated from healthy human peripheral blood expressed CRTH2, a smaller proportion of ILC2s isolated from nondiseased human lung expressed CRTH2, suggesting that dynamic regulation of CRTH2 expression might be associated with the migration of ILC2s into tissues. Consistent with this, murine ILC2s expressed CRTH2, migrated toward PGD2 in vitro, and accumulated in the lung in response to PGD2 in vivo. Furthermore, mice deficient in CRTH2 exhibited reduced ILC2 responses and inflammation in a murine model of helminth-induced pulmonary type 2 inflammation. Critically, adoptive transfer of CRTH2-sufficient ILC2s restored pulmonary inflammation in CRTH2-deficient mice. Together, these data identify a role for the PGD2-CRTH2 pathway in regulating the in vivo accumulation of ILC2s and the development of type 2 inflammation in the lung.
Figures
Similar articles
-
The Prostaglandin D2 Receptor CRTH2 Promotes IL-33-Induced ILC2 Accumulation in the Lung.J Immunol. 2020 Feb 15;204(4):1001-1011. doi: 10.4049/jimmunol.1900745. Epub 2020 Jan 3. J Immunol. 2020. PMID: 31900341 Free PMC article.
-
Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells.J Allergy Clin Immunol. 2014 Apr;133(4):1184-94. doi: 10.1016/j.jaci.2013.10.056. Epub 2013 Dec 31. J Allergy Clin Immunol. 2014. PMID: 24388011 Free PMC article.
-
Cytokine-induced endogenous production of prostaglandin D2 is essential for human group 2 innate lymphoid cell activation.J Allergy Clin Immunol. 2019 Jun;143(6):2202-2214.e5. doi: 10.1016/j.jaci.2018.10.069. Epub 2018 Dec 19. J Allergy Clin Immunol. 2019. PMID: 30578872
-
Targeting the PGD2/CRTH2/DP1 Signaling Pathway in Asthma and Allergic Disease: Current Status and Future Perspectives.Drugs. 2017 Aug;77(12):1281-1294. doi: 10.1007/s40265-017-0777-2. Drugs. 2017. PMID: 28612233 Free PMC article. Review.
-
Antagonists of the prostaglandin D2 receptor CRTH2.Drug News Perspect. 2008 Jul-Aug;21(6):317-22. doi: 10.1358/dnp.2008.21.6.1246831. Drug News Perspect. 2008. PMID: 18836589 Review.
Cited by
-
Single-Cell Multiparametric Analysis of Rare HIV-Infected Cells Identified by Duplexed RNAflow-FISH.Methods Mol Biol. 2022;2407:291-313. doi: 10.1007/978-1-0716-1871-4_20. Methods Mol Biol. 2022. PMID: 34985672
-
Sensing of physiological regulators by innate lymphoid cells.Cell Mol Immunol. 2019 May;16(5):442-451. doi: 10.1038/s41423-019-0217-1. Epub 2019 Mar 6. Cell Mol Immunol. 2019. PMID: 30842626 Free PMC article. Review.
-
Innate immune cell dysregulation drives inflammation and disease in aspirin-exacerbated respiratory disease.J Allergy Clin Immunol. 2021 Aug;148(2):309-318. doi: 10.1016/j.jaci.2021.06.016. J Allergy Clin Immunol. 2021. PMID: 34364539 Free PMC article. Review.
-
Innate lymphoid cells and allergic disease.Ann Allergy Asthma Immunol. 2017 Dec;119(6):480-488. doi: 10.1016/j.anai.2017.08.290. Ann Allergy Asthma Immunol. 2017. PMID: 29223298 Free PMC article. Review. No abstract available.
-
Innate Lymphoid Cells (ILCs) as Mediators of Inflammation, Release of Cytokines and Lytic Molecules.Toxins (Basel). 2017 Dec 10;9(12):398. doi: 10.3390/toxins9120398. Toxins (Basel). 2017. PMID: 29232860 Free PMC article. Review.
References
-
- Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nature Immunology. 2013;14(6):536–542. - PubMed
-
- Spits H, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nature Reviews Immunology. 2013;13(2):145–149. - PubMed
-
- Koyasu S, Moro K. Innate Th2-type immune responses and the natural helper cell, a newly identified lymphocyte population. Current Opinion in Allergy and Clinical Immunology. 2011;11(2):109–114. - PubMed
-
- Barlow JL, McKenzie AN. Nuocytes: expanding the innate cell repertoire in type-2 immunity. Journal of Leukocyte Biology. 2011;90(5):867–874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI074878/AI/NIAID NIH HHS/United States
- T32-AI060516/AI/NIAID NIH HHS/United States
- U01-AI095608/AI/NIAID NIH HHS/United States
- R01 AI095466/AI/NIAID NIH HHS/United States
- AI061570/AI/NIAID NIH HHS/United States
- P01 AI106697/AI/NIAID NIH HHS/United States
- F32-AI098365/AI/NIAID NIH HHS/United States
- T32 AI007532/AI/NIAID NIH HHS/United States
- K08-DK093784/DK/NIDDK NIH HHS/United States
- AI102942/AI/NIAID NIH HHS/United States
- AI106697/AI/NIAID NIH HHS/United States
- AI074878/AI/NIAID NIH HHS/United States
- T32-AI007532/AI/NIAID NIH HHS/United States
- AI087990/AI/NIAID NIH HHS/United States
- F31 AG047003/AG/NIA NIH HHS/United States
- T32 AI060516/AI/NIAID NIH HHS/United States
- R01 AI102942/AI/NIAID NIH HHS/United States
- R21 AI087990/AI/NIAID NIH HHS/United States
- AI095608/AI/NIAID NIH HHS/United States
- P30 CA016520/CA/NCI NIH HHS/United States
- P30-CA016520/CA/NCI NIH HHS/United States
- K08 DK093784/DK/NIDDK NIH HHS/United States
- F32 AI098365/AI/NIAID NIH HHS/United States
- U01 AI095608/AI/NIAID NIH HHS/United States
- R01 AI061570/AI/NIAID NIH HHS/United States
- R01 AI097333/AI/NIAID NIH HHS/United States
- AI095466/AI/NIAID NIH HHS/United States
- F31-AG047003/AG/NIA NIH HHS/United States
- AI097333/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

